A Meta-Analysis of Vagus Nerve Stimulation in Patients with Lennox Gastaut Syndrome
Abstract number :
1.457
Submission category :
3. Neurophysiology / 3E. Brain Stimulation
Year :
2018
Submission ID :
544022
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Maxine Dibue-Adjei, Medical Faculty Heinrich Heine University; Teresa Greco, LivaNova Italia Italia S.r.l.; Hans-Jakob Steiger, Medical Faculty Heinrich Heine University; and Marcel Alexander Kamp, Medical Faculty Heinrich Heine University
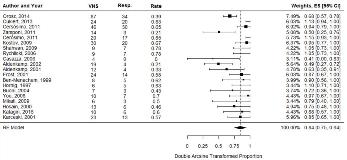
Rationale: Lennox-Gastaut Syndrome (LGS) is among the most severe epileptic encephalopathies and accounts for 5–10% of children with seizures. Optimal LGS management requires complex anti-epileptic strategies combining AED polypharmacy and non-pharmacological treatments while balancing side-effects and preserving cognitive function. A recently published expert consensus recommends early integration of non-pharmacological treatments including vagus nerve-stimulation (VNS) into LGS treatment strategies. A meta-analysis was therefore conducted to assessed efficacy of VNS in patients with LGS. Methods: BioMedCentral, PubMed and Embase literature databases were searched for pertinent studies. Articles were assessed by two trained investigators, with divergences resolved by consensus. The primary was the proportion of responders (>50% seizure frequency reduction) of the total LGS patients treated with VNS. Explorative analyses were performed on the number of subjects experiencing adverse events. Pooled estimate was derived by using a random effect model. Freeman-Tukey double arcsine transformation was used to normalize the proportions. Restricted Maximum-Likelihood estimator has been used to estimate the between-studies variance. Univariate meta-regression analyses and stratified meta-analyses were performed to explore the relation between study-specific responder proportions and year of publication, longest follow-up available and design. Results: Data from 388 patients in 21 clinical studies were analyzed (Fig.1). The pooled proportion of responders was 0.84 [95% CI: 0.75, 0.94], with 20 studies included (Table 1). Absence of publication bias on responder proportions was confirmed by the rank correlation Begg’s test (p-value =0.6) and the regression Egger’s test (p-value =0.8). Presence of heterogeneity (I2 =63%; Q p-value <0.001) was not explained by year of publication (n =20, slope coefficient= 0.006; 95% CI: -0.001, 0.024; p-value= 0.7) or length of study follow-up (n =20, slope coefficient= -0.0003; 95% CI: -0.005, 0.004]; p-value= 0.9), respectively (Fig. 2). Responder rate was also not affected by study design: n=9, proportion =0.88 [95% CI: 0.75, 1.00] and n=10, proportion =0.81 [95% CI: 0.66, 0.97] in the retrospective and prospective studies respectively (Fig. 3a and b). The pooled proportion of subject with serious or mild/transient adverse events was 0.07 [95% CI: 0.02, 0.012] with 12 studies included (I2 =41%; Q p-value =0.07) and 0.41 [95% CI: 0.14, 0.67] with 7 studies included (I2 =92%; Q p-value <0.001) respectively (Table 2). Conclusions: This meta-analysis suggests that VNS is an effective adjunctive treatment for LGS patients resulting in a responder proportion of 0.84. Further studies will be needed to confirm the finding of potential superior efficacy of VNS in LGS populations compared to heterogeneous drug-resistant epilepsy populations. Funding: This study was funded by LivaNova PLC.
.tmb-.jpg?Culture=en&sfvrsn=696919c_0)