A Phase 1 Single Ascending Dose, Multiple Dose, and Food Effect Trial in Healthy Volunteers
Abstract number :
1.300
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
500435
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Kevan VanLandingham, Greenwich Biosciences, Inc.; Barry E. Gidal, University of Wisconsin School of Pharmacy; Graham Blakey, Consult2deliver Ltd; Lesley Taylor, GW Research Ltd; and Gilmour Morrison, GW Research Ltd
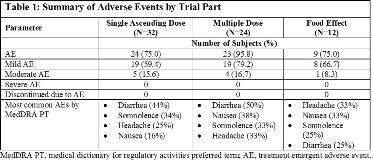
Rationale: A pharmaceutical formulation of highly purified cannabidiol oral solution (CBD) has been studied for add-on treatment in patients with Lennox-Gastaut and Dravet syndromes. This trial assessed the safety, tolerability, and pharmacokinetics (PK) of this formulation of CBD in healthy volunteers, as well as the effects of food on exposure. Methods: The trial consisted of 3 arms, as follows:--Single ascending dose (SAD): Single oral doses of 1500, 3000, 4500, or 6000 mg CBD (n=6/group) or placebo (n=2/group).--Multiple dose (MD): Multiple oral doses of 750 or 1500 mg CBD (n=9/group) or placebo (n=3/group) twice-daily (b.i.d.) for 6 days with a single dose on Day 7.--Food effect (FE): A single oral 1500 mg CBD dose fed and fasted (n=12).Plasma concentrations of CBD and its metabolites (6-hydroxy-cannabidiol [6-OH-CBD], 7-hydroxy-cannabidiol [7-OH-CBD] and 7-carboxy-cannabidiol [7-COOH-CBD]) were determined using validated bioanalytical methods. Safety parameters were monitored throughout the trial. Results: After a single oral administration, CBD appeared rapidly in plasma with a tmax of approximately 4–5 hours. The major circulating metabolite was 7-COOH-CBD (~95% of all drug-related material). The metabolite, 7-OH-CBD, was present at around half the CBD concentration, and 6-OH-CBD was a relatively minor metabolite. Plasma exposure to CBD (Cmax and AUC0-t) increased with dose in a slightly less than dose-proportional manner (Cmax slope: 0.73 [90% CI: 0.35, 1.12]; AUC0-t slope: 0.64 [90% CI: 0.27, 1.01]). CBD elimination was multi-phasic and the majority of CBD AUC was described within 12 hours post-dose. CL/F of CBD was high (1111-1909 L/h) and Vz/F was large (20963-42849 L). CBD reached steady state after approximately 2 days with moderate accumulation (1.8 to 2.6-fold) after 750 and 1500 mg CBD b.i.d. On Day 7, a 2-fold increase in CBD dose resulted in 1.6- and 1.9-fold increase in geometric mean Cmax and AUCtau, respectively. The terminal elimination t½ (approx. 60 hours) overestimates CBD elimination and can mislead drug accumulation upon multiple dosing. The calculated effective t½ more clearly describes the rate of drug accumulation and systemic removal across the entire dosing interval, providing a better understanding of CBD clearance at steady state and potential drug dosing intervals. Therefore the effective t½ is likely to be substantially lower. Cmax was 330 ng/mL and AUCtau was 1745 ng·h/mL after 750 mg CBD b.i.d. A high fat meal increased plasma exposure to CBD by 4.85-fold (90% CI: 4.01, 5.87) for Cmax and 4.2-fold (90% CI: 3.63, 4.85) for AUC0-t with no effect on tmax or t½. CBD was generally well tolerated; most adverse events (AEs) were mild and none were severe or serious (Table 1). Conclusions: After multiple b.i.d. dosing of orally administered CBD, parent drug levels appeared rapidly in plasma. Exposure increased in a slightly less than dose-proportional manner and was over 4-fold higher for fed vs fasted. CBD was generally well tolerated over the dose range studied, with AEs being mild/moderate in severity. Funding: GW Research Ltd