ABERRANT HIPPOCAMPAL NEUROGENESIS IS REQUIRED TO DRIVE EPILEPSY AND ASSOCIATED COGNITIVE DECLINE
Abstract number :
2.368
Submission category :
Year :
2014
Submission ID :
1868920
Source :
www.aesnet.org
Presentation date :
12/6/2014 12:00:00 AM
Published date :
Dec 4, 2014, 06:00 AM
Authors :
Kyungok Cho, Zane Lybrand, Naoki Ito, Rebecca Brulet, Farrah Tafacory, Ling Zhang, Levi Good, Kerstin Ure, Steven Kernie, Shari Birnbaum, Helen Scharfman, Amelia Eisch and Jenny Hsieh
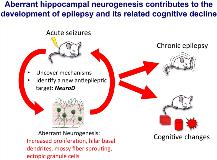
Rationale: Epileptic seizures trigger aberrant hippocampal neurogenesis. However, the functional role of adult-generated neurons in the development of epilepsy or associated cognitive deficits remains to be determined. Methods: To investigate the role of aberrant neurogenesis in the generation of recurrent seizures, we took advantage of Nestin-δ-HSV-thymidine kinase-EGFP (Nestin-TK) transgenic mice to achieve ablation of adult hippocampal neurogenesis. This genetic model selectively ablates dividing neural stem/progenitors upon ganciclovir (GCV) administration, without affecting glial and endothelial cells. We then injected pilocarpine to induce epilepsy and examined spontaneous recurrent seizure frequency by video-EEG monitoring and hippocampal memory function by novel location and novel object recognition tests. To gain insight into the cell-autonomous mechanisms that promote aberrant neurogenesis, we utilized Nestin-CreERT2;NeuroDloxP/loxP;Rosa26(R26R)-YFP (cKO) mice and Nestin-CreERT2;NeuroD+/+;R26R-YFP (WT) mice, to delete NeuroD in nestin-expressing stem cells and their progeny after tamoxifen injection. Then, kainic acid was injected to induce acute seizures and seizure-induced aberrant hippocampal neurogenesis was evaluated by a variety of stage-specific markers. Results: We found that ablation of adult neurogenesis prior to acute seizures reduced the frequency of recurrent seizures and normalized epilepsy-associated cognitive deficits. Ablation of neurogenesis alleviated aberrant hippocampal neurogenesis, in particular, the production of ectopic granule cells. Furthermore, we identified the basic helix-loop-helix proneural transcription factor NeuroD to be essential for seizure-induced aberrant hippocampal neurogenesis. Strikingly, a single ablation of adult neurogenesis continued to suppress spontaneous seizure frequency for over a year. Conclusions: These findings highlight a causal role of neurogenesis in the generation of epilepsy and suggest that strategies designed to block adult hippocampal neurogenesis, specifically, NeuroD-expressing cells, may have therapeutic potential for reducing spontaneous seizure formation. Our studies also provide a cautionary note regarding neuroregenerative approaches whereby addition of aberrant neurogenesis may exacerbate rather than mitigate disease symptoms.