ANTICONVULSANT DRUG CONCENTRATIONS IN CAPILLARY SAMPLES COLLECTED ONTO FILTER PAPER
Abstract number :
2.380
Submission category :
Year :
2005
Submission ID :
5687
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
Jennifer A. Collins, Karla J. Walker, and Gregory C. Janis
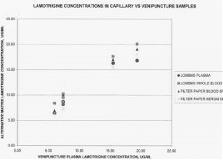
Therapeutic Drug Monitoring (TDM) of anticonvulsants is an important tool in the management of patients with seizure disorders. We have previously reported the development of an analytical method for these compounds utilizing HPLC coupled to tandem mass spectrometry (LCMSMS). This comprehensive method can analyze up to 13 anticonvulsant drugs from a single sample using minimum sample volume. The sensitivity of the LCMSMS method allows us to propose the use of a filter paper collection technique for monitoring anticonvulsant drug levels. This would provide a means for caregivers to collect TDM samples at crucial time points in non-medical settings, as well as facilitate routine sample collection. We have recently initiated a study protocol with a clinical practice group to establish relative equivalence of the anticonvulsant concentrations in each matrix and to validate the feasibility of the alternative collection/testing paradigm. Preliminary data is presented. Subjects enrolled in the study adhere to the monitoring schedule prescribed by treating physicians. Venipuncture samples are collected in the clinic. A lancet is then used to pierce the finger and capillary blood is collected onto a Filter Paper (FP) blood collection device and a serum separator FP device. Specimens are transported to the laboratory for testing. Specimens tested for the comparison are: venous whole blood, venous plasma, capillary whole blood on FP and capillary plasma on FP. The plasma sample collected by venipuncture is considered the predicate sample/method for comparison purposes.
In the laboratory, the filter paper specimens are processed for testing by punching circles from the filter paper and placing them into test tubes. Internal standards are added and the analytes are solubilized into a mixture of acetonitrile and water. Quantitative standards and controls are processed the same manner. Processed samples, standards and controls are analyzed by LCMSMS using the methods developed for the target compounds. Preliminary results for one of the monitored compounds, Lamotrigine (LamictalR) are presented in Figure 1. This data represents a limited number of sampling points (n = 6); the study protocol is designed for 20 or more comparisons for each compound. Linear regression statistics indicate correlation coefficients [gt] 0.956 for each matrix.[figure1] This preliminary data demonstrates excellent correlation of lamotrigine concentrations in samples obtained using alternative sampling techniques. As the study progresses, additional data will allow for more rigorous comparisons for the included anticonvulsant compounds. (Supported by MEDTOX Laboratories, Inc.)