ARE FDA BIOEQUIVALENCE STANDARDS SUFFICIENT FOR [ldquo]NARROW THERAPEUTIC RATIO[rdquo] AED[apos]S: PHARMACOKINETIC COMPARISONS OF EXTENDED-RELEASE AND IMMEDIATE-RELEASE CARBAMAZEPINE FORMULATIONS?
Abstract number :
1.272
Submission category :
Year :
2003
Submission ID :
484
Source :
www.aesnet.org
Presentation date :
12/6/2003 12:00:00 AM
Published date :
Dec 1, 2003, 06:00 AM
Authors :
Gregory L. Krauss, Hamzeh Fayez, Miller Akemi Department of Neurology, Johns Hopkins University, Baltimore, MD; Department of Clinical Pharmacology, Johns Hopkins University, Baltimore, MD
FDA bioequivalence standards may miss clinically important differences in drug delivery for AEDs that have narrow therapeutic ratios (NTR). An example of the limitations of the current standards is the observation that many patients treated with extend-release carbamazepine (ER-CBZ; Carbatrol ) have fewer CNS side-effects than patients treated with immediate-release carbamazepine (IR-CBZ). The differences in tolerability between these formulations may be related to intra- and inter-individual variability of absorption and concentrations over time. We compared pharmacokinetic (PK) studies of ER-CBZ and IR-CBZ to see whether these differences may be missed by current bioequivalence standards.
The pharmacokinetic (PK) profile of ER-CBZ (Carbatrol) was modeled using individual data from three single-dose studies of Carbatrol provided by Shire Pharmaceuticals (Shire studies 107, 108, 112). The PK profile of IR-CBZ was modeled using data from 10 published studies. Steady-state elimination rate constant (K[sub]el[/sub]) and absorption rate constant (K[sub]a[/sub]) for Carbatrol and IR-CBZ were determined and used for PK modeling. We performed pharmacokinetic modeling for both formulations using a one-compartment model with a lag absorption phase (WinNonlin pharmacokinetic software). The K[sub]el[/sub] for Tegretol and Carbatrol were not significantly different and therefore the mean K[sub]el[/sub] value of 0.04 was used to simulate drug delivery and produce the PK profile of both formulations.
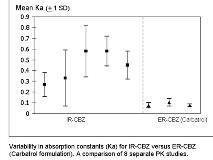
Absorption constants (K[sub]a[/sub]) for IR-CBZ derived from published PK studies were five-fold (Ka mean 0.27 to 0.6) those for ER-CBZ (Carbatrol) (Ka mean 0.07 to 0.10) reflecting the extended-release properties of Carbatrol. Individual variability in absorption was markedly increased, however, for IR-CBZ compared with Carbatrol: the standard deviation for individual absorption for IR-CBZ was approximately five times that of ER-CBZ (Carbatrol). Pharmacokinetic modeling demonstrated similar total AUC/24 hr for both formulations; however, fluctuations in daily concentrations (C[sub]max[/sub] [ndash] C[sub]min[/sub])/C[sub]av[/sub] for IR-CBZ TID were twice those for ER-CBZ BID.
Individual absorption of IR-CBZ is highly variable compared to ER-CBZ and probably accounts for improved tolerability of ER-CBZ compared to IR-CBZ. Current FDA bioequivalence standards do not recognize the importance of uniform delivery for AEDs with narrow therapeutic ratios.[figure1]
[Supported by: Shire Pharmaceuticals, US]