Changes in Seizure Rates During Pregnancy: Results from the MONEAD Study
Abstract number :
3.229
Submission category :
4. Clinical Epilepsy / 4E. Women
Year :
2018
Submission ID :
502087
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Page B. Pennell, Harvard Medical School, Brigham and Women's Hospital; Kimford J. Meador, Stanford University; Ryan C. May, Emmes Corporation; Jacqueline A. French, New York University; Elizabeth Gerard, Northwestern University Feinberg School of Medicine
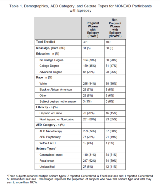
Rationale: The AAN/AES Pregnancy Parameters concluded that there is insufficient evidence to determine if seizure frequency changes occur in pregnant women with epilepsy (PWWE), because the appropriate gold standard comparison group, non-pregnant WWE (NPWWE), has not been employed. Methods: The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study is a prospective, observational, multi-center investigation of pregnancy outcomes for both the mother and child. Inclusion criteria included ages 14-45 years, and <20 weeks gestational age for pregnant groups. Exclusion criteria included history of psychogenic non-epilepsy seizures, IQ<70, other major medical illness, progressive cerebral disease, and history of switching AEDs prior to enrollment for PWWE. NPWWE were enrolled to maintain a group balance with PWWE on age, race, ethnicity, baseline seizure frequency and AED regimen. Detailed epilepsy classification was performed at enrollment, with review by the semiology core. Seizures were recorded prospectively with an electronic daily diary and verified at each study visit. For PWWE, the number of seizures prior to enrollment but after conception were included in the pregnancy seizure rates. Six weeks following delivery to 9 months postpartum was employed as non-pregnant baseline seizure frequency in the PWWE group, since it was collected prospectively. A comparable time interval was used for the NPWWE as baseline seizure frequency. This preliminary report analyzed changes in total seizures and number of days with seizures per month (30 days) compared to non-pregnant baseline in each trimester in the PWWE group, and during comparable time intervals in the NPWWE group, utilizing Wilcoxon signed rank test. Wilcoxon rank sum test was used for comparison between PWWE and NPWWE. Results: MONEAD enrolled 351 PWWE, 109 NPWWE, and 105 healthy pregnant women not reported on here. Demographics, AED category and seizure types are listed in Table 1. Significant increases in monthly rates of total seizures and days with seizures occurred in the 2nd trimester in PWWE compared to non-pregnant baseline, and no significant changes occurred in the NPWWE (Table 2). Comparison of PWWE and NPWWE did not show any differences. Conclusions: The PWWE group experienced increased monthly seizure rate and days with seizures during the second trimester compared to the non-pregnant baseline, whereas non-pregnant women with epilepsy did not have an increase during comparable intervals. However, direct comparisons of PWWE and NPWWE were not significant. Future analyses will focus on seizures that impair awareness and include percentage of women with seizure worsening; secondary analyses will include evaluation of convulsive seizures only, loss of seizure-free status, and determination of risk factors for increased seizures during pregnancy (e.g., AED employed and blood levels, epilepsy/seizure types, non-compliance, and sleep deprivation). Funding: National Institutes of Health, NINDS and NICHD #U01-NS038455, U01-NS050659.
.tmb-.png?Culture=en&sfvrsn=14250b28_0)