Clinical Factors Associated With the Use of Infusion Therapy in Pediatric Status Epilepticus: A Case Control Study (the pSERG Consortium)
Abstract number :
3.197
Submission category :
4. Clinical Epilepsy / 4B. Clinical Diagnosis
Year :
2018
Submission ID :
507294
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Ravindra Arya, Cincinnati Children's Hospital Medical Center, University of Cincinnati; Katrina Peariso, Cincinnati Children's Hospital Medical Center, University of Cincinnati; Tracy A. Glauser, Cincinnati Children's Hospital Medical Center, University o
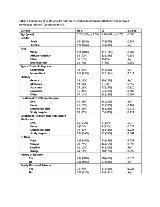
Rationale: To identify clinical factors that may be obtained early in the presentation of pediatric status epilepticus (SE) which are associated with the use of continuous infusion (CI) anti-seizure medications (ASM). Methods: Data from June 2011 – February 2018 were extracted from the multicenter pediatric Status Epilepticus Research Group (pSERG) study. Common inclusion criteria for our cohort with prolonged convulsive seizures and refractory SE consist of: 1) age 1 month to 21 years; 2) convulsive seizures at onset; 3) application of 2 or more ASM for convulsive seizures, and 4) consent obtained and assent obtained, when applicable. We excluded patients with: 1) non-convulsive SE detected on EEG (without convulsive seizures at onset); 2) non-convulsive SE and infrequent myoclonic jerks, and 3) unable to obtain consent/assent. Demographic and clinical variables were used to assess the use of CI ASMs for control of refractory SE. Patient’s receiving CI ASM were compared with patients who did not receive CI ASM. Univariate logistic regressions were performed for each clinical determinant, utilizing the use of CI ASM as the dependent variable, and an optimal multivariable model was then derived. Results: This data set included information from 171 patients (57% male) where CI ASM were used for treatment of SE and 268 patients (54% male) that did not receive CI ASM for treatment of SE (Table 1). For patients where CI was used versus those where it was not, there were significant differences on univariate logistic regression in SE type, first non-benzodiazepine (BZD) ASM and a family history of seizures. There was also a trend toward significance in patient age, with younger patients less likely to receive a continuous infusion (Table 1). A multivariable logistic regression model was designed using variables that had p-values = 0.1 on univariate logistic regression modelling and would be available proximate to the onset of SE (SE type, non-BZD ASM administration, patient age, family history of seizure, and CT head imaging). The optimal model found the location of the first non-BZD ASM, CT head acquisition, a family history of seizures and patient’s age to be associated with the use of CI ASMs. Having received the first non-BZD ASM in a hospital compared with an EMS setting demonstrated an increase in the use of CI ASMs (OR 3.4-3.7 for outside hospital and study hospital, respectively), while not having a head CT, and a family history of seizures were associated with a decreased use of CI ASMs (OR of 0.58 and 0.64, respectively). (Table 2). There was no statistically significant association with patient age in this model. Conclusions: Administration of the first non-BZD ASM in a hospital was positively associated with the use of CI ASMs for treatment of SE, while not having a CT head scan and having a family history of seizures are negatively associated with the use of CI for treatment of SE. Future studies will specifically address if the first non-BZD ASM location, CT head scan on presentation and family history of seizures can provide for accurate stratification of patients into two separate treatment pathways for SE. Funding: Pediatric Epilepsy Research Foundation and the Epilepsy Research Fund
.tmb-.jpg?Culture=en&sfvrsn=ad002381_0)