Clobazam Efficacy, Tolerability and Adverse Event in 367 Saudi Children With Intractable Epilepsy
Abstract number :
1.319
Submission category :
7. Antiepileptic Drugs / 7E. Other
Year :
2018
Submission ID :
481799
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Suad Alyamani, King Faisal Specialist Hospital and Research Centre; Safinaz Alharthi, King Faisal Specialist Hospital and Research Centre; Ashwaq Alsulami, King Faisal Specialist Hospital and Research Centre; Edward De Vol, King Faisal Specialist Hospital
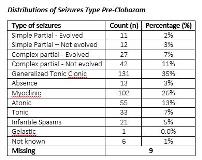
Rationale: Clobazam is a unique 1,5 benzodiazepine which had been used to treat intractable epilepsy for decades. We treated 367 patients with intractable epilepsy with Clobazam and will report on efficacy and tolerability. Methods: Retrospective chart review of 367 patient treated with Clobazam for intractable epilepsy. The information was collected in case report from (CRF) and entered into RedCap (database). Data included: age, sex, weight, seizure types and frequency, etiology of seizure, concomitant anti-convulsants, seizure frequency, pre and post Clobazam, Clobazam dose and duration of treatment, Clobazam side effects and discontinuation rate and reason of discontinuation.Descriptive statistics were used to summarize the patient baseline characteristics case, gender, event frequencies of types pre and post seizure, the monthly average of seizures pre and post-trial, for quantifying etiology and adverse events. Chi-squared test, Bivariate and Bivariate Fisher’s exact test were also used. Results: Male 208 and female 159. Age range from 7 months to 34 years with a mean age of 11.8 years. Type of seizures (Table 1). All seizures responded to Clobazam except Gelastic Seizure (Table 2). The average effective dose is 0.9mg/Kg/day. Adverse effects were seen in 20 out of 367 (5%). The most common adverse effects is sedation (3.54%). The side effect were mild to moderate in severity. Discontinuation rate was 2%. Conclusions: Clobazam showed high efficacy in almost all seizure types in our group of patients. Clobazam was tolerated well with low adverse event, only 5% and discontinuation rate of 2%. Funding: This is a retrospective study; therefore funding is not needed.
.tmb-.jpg?Culture=en&sfvrsn=e6537a6_0)