Disordered Breathing and Premature Death in a Mouse Model of Dravet Syndrome
Abstract number :
3.157
Submission category :
3. Neurophysiology / 3F. Animal Studies
Year :
2018
Submission ID :
502544
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Fu-Shan Kuo, University of Connecticut and Daniel Mulkey, University of Connecticut
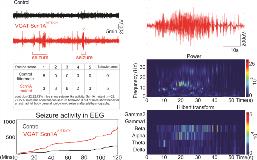
Rationale: : Dravet syndrome (DS) is an early onset form of epilepsy with a high rate of sudden unexpected death in epilepsy (SUDEP). Although cardiac failure is considered a leading cause of death in DS, respiratory dysfunction can precipitate cardiac failure, and a recent study showed that DS patients commonly exhibit breathing problems and apnea. Therefore, we hypothesize that respiratory dysfunction contributes to SUDEP in DS. Further, most DS cases (~95%) result from mutations in the Scn1A gene that encodes a voltage-dependent sodium channel 1.1. Evidence also suggests that loss of Scn1A preferentially disrupts the function of inhibitory neurons, and since inhibitory neurons control many aspects of breathing including the CO2/H+-dependent drive to breathe (i.e. respiratory chemoreception), we wanted to determine if loss of Scn1A function in inhibitory neurons affects cellular properties of chemosensitive neurons and the drive to breathe. Methods: We chose to model DS by using the cre-lox system to express a human DS-associated loss-of-function Scn1A mutation (A1783V) selectively in inhibitory neurons . Cortical EEG electrodes were used to monitor seizure activity in Scn1AA1783/+ and littermate controls posnatal 15 days, just prior to onset of premature death. At the cellular level, we use electrophysiological techniques to characterize intrinsic electrical properties and CO2/H+-sensitivity of chemosensitive neurons and inhibitory neurons located in a brainstem respiratory center known as the retrotrapezoid nucleus (RTN) in brain slices from control and Scn1AA1783V/+ mice. At the whole animal level, we use whole-body plethysmography to assess baseline breathing and the ventilatory response to CO2 in control and Scn1AA1783V/+ mice. All experiments are performed in neonatal pups (10-15 days of age) results are analyzed by t-test or two-way RM-ANOVA and Tukey’s multiple comparison test as appropriate.(p < 0.05). Results: Scn1AA1783V/+ mice show frequent spontaneous high frequency (beta) and amplitude spike activity indicative of seizure-like activity (n=22 for both genotype; pA1783V/+ mice also show complete lethality at ~22 days of age, therefore, we consider this a reasonable model of SUDEP in DS. Scn1AA1783V/+ mice also show hypoventilation and apnea under room air conditions and a blunted ventilatory response to CO2 (control n=16, Scn1A A1783V/+ n=22; pA1783V/+ were less excitable (control n=26, Scn1A A1783V/+n=36; p<0.01), whereas excitatory chemosensitive neurons showed an increase in baseline activity and CO2/H+-sensitivity compared to control cells. Conclusions: These results provide the first characterization of breathing in a mouse model of DS and since these mice die prematurely, we consider this a useful model to study mechanisms contributing to SUDEP in DS. We show that Scn1A
.tmb-.jpg?Culture=en&sfvrsn=5e8f632c_0)