Dissecting the Heterogeneous Clinical Response to Padsevonil in Adults with Drug-Resistant Focal Seizures: Post hoc Responder Analysis of a Proof-of-Concept Trial
Abstract number :
1.289
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
496151
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Wim Van Paesschen, University Hospital Leuven; Manuel Toledo, Epilepsy Unit, Vall d'Hebron University Hospital; Christian Brandt, Bethel Epilepsy Centre; Bernhard J. Steinhoff, Kork Epilepsy Centre; Marian Majoie, Academic Centre for Epileptology Kempenha
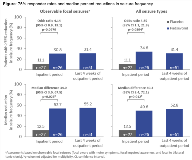
Rationale: Padsevonil (PSL; UCB0942) is the first rationally designed antiepileptic drug (AED) candidate combining presynaptic activity (equally high affinity to all three synaptic vesicle 2 isoforms) with postsynaptic enhancement of GABAergic inhibition (moderate affinity at benzodiazepine site of GABAA receptor). PSL showed activity across 10 animal models of focal and generalized seizures, and in the amygdala kindling model of epilepsy. Methods: A phase II proof-of-concept trial (EP0069; NCT02495844) enrolled adults (≥18 years) with ≥4 observable focal seizures per week, who had failed to achieve seizure control with ≥4 AED schedules of adequate dose and duration, and were on a stable dose of ≥1 AED. The trial consisted of a 3-week double-blind, placebo (PBO)-controlled Inpatient Period, and an 8-week open-label Outpatient Period during which all patients received PSL (800 mg/day). Primary outcome was 75% responder rate (≥75% reduction in focal seizure frequency) from Baseline to 2-week Inpatient maintenance. A post hoc responder analysis (univariate and multivariate) was conducted to determine whether key clinical variables had an effect on seizure responses (75% responder rate, median percent reduction). Results: 55 patients were randomized (PSL: 28; PBO: 27). At Baseline, 50% of patients had ≥8 observable focal seizures per week (all seizures: ≥10) and 75% had ≥8 prior AEDs (past and concomitant). Assessments of observable focal seizures and of all seizures showed higher 75% responder rates and median percent reductions in seizure frequency for PSL vs PBO during the Inpatient Period, with efficacy maintained throughout the Outpatient Period (Figure). The most common treatment-emergent adverse events with PSL were somnolence, dizziness, headache, and fatigue (Table). Due to the small sample size, the responder analysis could only detect variables with a large effect size. In the univariate analyses, patients with a higher number of prior AEDs, a history of epilepsy surgery, or psychiatric events during PSL treatment were less likely to respond to PSL. Those with an epilepsy duration of >20 years and age of onset of 15–20 years displayed higher responses. Concomitant use of levetiracetam, oxcarbazepine, lacosamide, lamotrigine, or valproate appeared not to influence response. In the multivariate analysis, general linear models with clinical factor selection highlighted the following significant factors contributing to seizure reduction during the Outpatient Period: number of prior AEDs, history of epilepsy surgery, psychiatric events during treatment, and history of status epilepticus. Conclusions: In this severely affected epilepsy population, PSL was associated with a meaningful reduction in seizure frequency. Post hoc preliminary analyses identified clinical factors related to PSL response despite the small sample size. These findings have to be confirmed in a larger dataset. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=c3565f81_0)