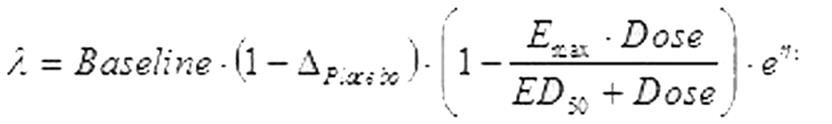DOSE-RESPONSE POPULATION ANALYSIS OF Keppra[trade] ADD-ON TREATMENT IN EPILEPTIC PATIENTS WITH PARTIAL ONSET SEIZURES
Abstract number :
1.337
Submission category :
Year :
2004
Submission ID :
4365
Source :
www.aesnet.org
Presentation date :
12/2/2004 12:00:00 AM
Published date :
Dec 1, 2004, 06:00 AM
Authors :
1Eric Snoeck, and 2Armel Stockis
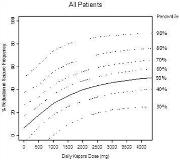
The aim of this population analysis was to describe the individual change in seizure frequency from baseline after treatment with Keppra (levetiracetam) or placebo and to model the dose-response relationship and assess the impact of potential covariates. Efficacy data from four double-blind, placebo-controlled parallel-group phase-III clinical trials were used. The final dataset used for the modeling of the dose-response relationship contained 4218 data rows for 958 individual epileptic patients with partial onset seizures. In the final model, the change in weekly seizure frequency for the improving patients was described by the following equation:[figure1]in which the drug effect was assumed to be an additional effect on top of a placebo effect [Delta][italic][sub]Placebo[/sub][/italic]. The changes in seizure frequency in patients improving or deteriorating on placebo and in patients deteriorating on Keppra, were described by dose-independent models. The number of seizures in an individual patient was modeled as a Poisson process and the seizure frequency between patients was assumed to be log-normally distributed. Modeling was performed using NONMEM version V. The final model successfully converged to an E[sub]max[/sub] dose-response relationship (Figure). A typical improving patient treated with placebo is predicted to have an 11% decrease in the seizure frequency from baseline, compared to 45% increase for a typical deteriorating patient. The typical value of the maximal reduction in seizure frequency from baseline in improving patients after treatment with Keppra was estimated to be 72%. The typical value of ED[sub]50[/sub] (dose producing half of the maximum effect) was estimated to be 1408 mg/day. Neither gender nor race, body weight, age, or number of concomitant AEDs appeared to have an effect on the improvement or deterioration of patients.[figure2] Add-on treatment of Keppra demonstrates a dose-response relationship in approximately 75% of the patients with refractory partial seizures. The current maximum recommended daily dose corresponds to twice the ED[sub]50[/sub] predicted by the model. (Supported by UCB Pharma SA)