Effect of Perampanel on EEG Clinical Idiopathic Generalized Convulsive Epilepsy, Continuous Spike-Wave in Sleep, and Lennox-Gastaut Syndrome
Abstract number :
2.031
Submission category :
3. Neurophysiology / 3C. Other Clinical EEG
Year :
2018
Submission ID :
501018
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Michael G. Chez, Sutter Neuroscience Institute
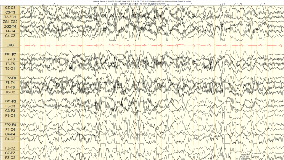
Rationale: Perampanel is a unique anticonvulsant acting as an AMPA receptor non-competitive antagonist. This medication has been shown to be useful in primary and primary generalized epilepsy in adults. Clinical experience and effect on EEG patterns in children who have generalized EEG discharges is not well known. This led to clinical trial of adding perampanel to pediatric patients with Lennox-Gastaut Syndrome ( LGS) and continuous Spike wave in sleep (CSWS) and idiopathic primary generalized epilepsy (PGE) in 6 pediatric patients. Observations of addition of perampanel to these patients medical regimen is reported. Methods: Six pediatric patients (female,11yr,PGE; male, 5yr, CSWS; 1female 8yr, 3 male, 6 yr, 11 yr, LGS) were studied based on clinical need for seizure control to add perampanel for clinical seizure control in patients with EEG showing presence of generalized or secondarily generalized Spike or poly-spike wave activity on EEG. Data reviewed included EEG before and after perampanel treatment to study the effect of this medication on the patient's seizure control and EEG patterns. Patients with LGS had Clobazam, valproic acid, and felbatol as comedications, CSWS case was clobazam, valproic acid, Zarontin, and new onset PGE case as first medication. EEG was collected with standard 10-20 arrays using Cadwel digital EEG. Average time between pre-/ post-EEG was 8-12 weeks. maximal dosage was 2-6 mg/day perampanel. Stable therapeutic levels for other medications were maintained between EEG samples. Results: Awake EEG interictal backgrounds were compared in these pediatric patients and the following findings occurred. PGE case normalization after 4 weeks of treatment at 2 mg/day and seizure freedom observed (Fig 1,2). No change on interictal EEg in LGS or CSWS cases was noted. Improved seizure control was seen in 2/4 LGS with clinical generalized convulsive seizures, but not absence or tonic seizures, and the PGE case. Conclusions: In pediatric patients with clinical generalized convulsive seizures, especially PGE, this observation suggest fycompa may improve interictal EEG. More clinical EEG data collection studies are warranted, Funding: No funding
.tmb-.png?Culture=en&sfvrsn=9cb2d2fb_0)