Effect of SCN1A Mutation Type on Cannabidiol (CBD) Response in Patients with Dravet Syndrome: Subgroup Analysis of Phase 3 Trial GWPCARE1
Abstract number :
1.299
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
500112
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Sameer M. Zuberi, University of Glasgow and Royal Hospital for Children; Joseph D. Symonds, Royal Hospital for Children, Glasgow; Andreas Brunklaus, University of Glasgow and Royal Hospital for Children; Claire Roberts, GW Research Ltd; Daniel Checketts,
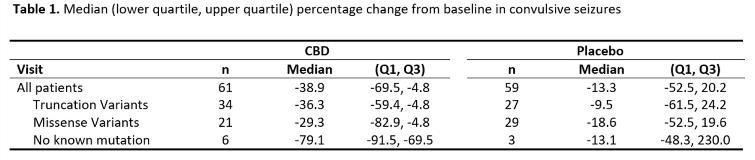
Rationale: A randomized controlled trial, GWPCARE1 (NCT02091375), showed that add-on treatment with a pharmaceutical formulation of highly purified CBD in patients with Dravet syndrome led to significant reductions in convulsive seizure frequency during the 14-week treatment period (39% on CBD vs 13% on placebo; p=0.01), with more adverse events for CBD than placebo (NEJM 2018; 376(21):2011–2020). Most patients with Dravet syndrome have a pathogenic SCN1A variant. A retrospective study has suggested that Dravet patients with truncating variants in SCN1A may respond differently to medications than those with missense variants (Epilepsia 2017; 58:282-290). Here we analyze whether SCN1A variant type was associated with CBD response in GWPCARE1. Methods: GWPCARE1 enrolled 120 patients (61 CBD; 59 placebo). Genetic information on SCN1A status was collected, and if unknown, then genetic analysis was offered. Probability of pathogenicity for each variant was assessed using Align, PolyPhen-2, SIFT, ClinVar, ExAC, gnomAD, and the published literature. For missense variants the associated functional domain of the channel was identified using Uniprot. CBD and placebo responses were then reevaluated in subgroups categorized by type of SCN1A variant. Results: Of 120 patients who participated in the trial, 111 (93%) had a pathogenic or likely pathogenic SCN1A mutation, either truncating variant (n=61) or missense variant (n=50); 9 (8%) had no known SCN1A mutation. Reductions in convulsive seizure frequency during the 14-week treatment period (primary endpoint) for patients treated with CBD were similar regardless of SCN1A variant (Table 1). There was no significant difference in the CBD treatment effect between patients with a truncating or missense variant for the primary endpoint (p=0.8866) or for any of the responder thresholds of =25% (p=0.9553), =50% (p=0.5981), or =75% (p=0.4058) reduction in convulsive seizure frequency. Of the patients with no known SCN1A mutation, six were randomized to CBD and three to placebo. Five out of the six treated with CBD had =50% reduction in convulsive seizure frequency while none of the three placebo patients did. In the overall cohort, incidence of adverse events was higher for CBD (93%) than placebo (75%), with most common being somnolence, diarrhea, and decreased appetite. Conclusions: The efficacy of CBD, as add-on therapy for the treatment of seizures associated with Dravet syndrome, does not appear to be influenced by either SCN1A status (positive or negative) or broad category of variant type (truncating or missense). Funding: GW Research Ltd.