Efficacy and Tolerability of Lacosamide and Controlled-Release Carbamazepine Monotherapy in Patients With Cerebrovascular Epilepsy Etiology: Post hoc Analysis of a Randomized Double-Blind Trial
Abstract number :
1.290
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
497183
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Reetta Kälviäinen, University of Eastern Finland and Kuopio Epilepsy Center; Ali Bozorg, UCB Pharma; Svetlana Dimova, UCB Pharma; Ying Zhang, UCB Pharma; Björn Steiniger-Brach, UCB Pharma; Bruno Ferrò, UCB Pharma; and Felix Rosenow, Ep
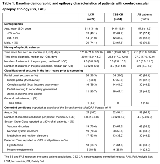
Rationale: The objective of this exploratory post hoc analysis was to assess the efficacy and tolerability of lacosamide (LCM) and controlled-release carbamazepine (CBZ-CR) monotherapy in adults with newly diagnosed epilepsy of cerebrovascular etiology. Methods: This exploratory post hoc analysis included patients with cerebrovascular epilepsy etiology randomized to LCM or CBZ-CR in a double-blind, non-inferiority monotherapy trial (SP0993; NCT01243177) in adults with newly diagnosed epilepsy. Patients with atrioventricular block or any other clinically relevant electrocardiographic abnormalities were excluded. LCM (initiated at 100mg/day) and CBZ-CR (initiated at 200mg/day) were titrated to 200mg/day or 400mg/day, respectively (first target dose level). Further dose increments were based on seizure control, approach closely reflecting clinical practice (dose level two LCM 400mg/day, CBZ-CR 800 mg/day; dose level three LCM 600 mg/day, CBZ-CR 1200 mg/day). Results: Cerebrovascular epilepsy etiology was reported in 61/886 (6.9%) treated patients (LCM n=27; CBZ n=34; mean age 57.6 years; 70.5% male; Table 1). Majority of patients remained on the first dose level (LCM 88.9%; CBZ-CR 67.6%); no patient on LCM escalated to the highest dose level. 66.7% patients on LCM and 50.0% on CBZ-CR completed the trial. The main reasons for discontinuation were adverse event (LCM 11.1%; CBZ-CR 20.6%) or lack of efficacy (LCM 3.7%; CBZ-CR 14.7%). A higher proportion of patients on LCM vs CBZ-CR completed 6 (81.5% vs 58.8%) and 12 (66.7% vs 50.0%) months of treatment without a seizure at the last evaluated dose level. The stratified Kaplan-Meier estimates of the proportion of patients with 6- and 12-month seizure-freedom at last evaluated dose were 95.5% and 91.1% on LCM and 82.0% and 75.1% on CBZ-CR, respectively. Efficacy analysis performed from the first dose of trial medication indicated 6- and 12-month seizure freedom rates of 66.7% and 55.6% on LCM and 47.1% and 38.2% on CBZ-CR, respectively. The stratified Kaplan-Meier estimates of the proportion of patients remaining seizure free for 12 months from the first dose of trial medication were 66.4% on LCM and 44.2% on CBZ-CR. Similar proportion of patients on LCM and CBZ-CR reported at least one treatment-emergent adverse event (TEAE) and lower proportion of patients on LCM experienced drug-related TEAEs (Table 2). The most frequently reported TEAEs on LCM were headache, dizziness and fatigue; on CBZ-CR: headache and dizziness. Conclusions: This exploratory post hoc analysis suggested that in patients with cerebrovascular epilepsy etiology, LCM monotherapy was efficacious and generally well tolerated with a discontinuation rate due to TEAEs similar to that observed in the overall LCM trial population. In this small subpopulation with cerebrovascular epilepsy etiology, LCM showed numerically better efficacy, lower incidence of drug-related TEAEs and discontinuation rates compared to CBZ-CR. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=366dd29a_0)