Expert Opinion: A New Treatment Algorithm for Lennox-Gastaut Syndrome (LGS) in Adult Patients
Abstract number :
3.201
Submission category :
4. Clinical Epilepsy / 4C. Clinical Treatments
Year :
2018
Submission ID :
504669
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Sanjeev V. Kothare, Zucker Hofstra School of Medicine/Northwell Health; Sami Aboumatar, Austin Epilepsy Care Center; David Burdette, Spectrum Health System; Ruben Kuzniecky, Zucker Hofstra School of Medicine/Northwell Health; Georgia Montouris, Boston Uni
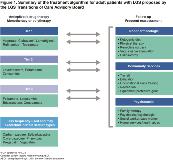
Rationale: LGS is characterized by multiple seizure types, abnormal electroencephalogram patterns, and intellectual disability. As patients mature, the transition of care from pediatric to adult neurologist is particularly relevant in the context of LGS because disconnects in treatment may arise, especially as many patients with LGS are refractory to antiepileptic drugs (AEDs). Current treatment algorithms are considered suboptimal for adults newly diagnosed with LGS and for patients with LGS undergoing transition of care from pediatric to adult services. Thus, a new treatment algorithm is needed. Methods: The LGS Transitions of Care Advisory Board met in November 2017 to discuss the development of a new treatment algorithm for adults with LGS. Collated responses to a pre-meeting survey of the AEDs most frequently selected to treat LGS in adults served as the basis for categorizing treatment preferences into Tiers 1–3. Results: Frequent reassessment of adult patients with LGS was considered a cornerstone of the proposed treatment algorithm. AEDs categorized into Tier 1 included those with FDA approval for LGS (clobazam, lamotrigine, rufinamide, and topiramate), and valproate. AEDs categorized into Tier 2 were those commonly used in the treatment of LGS including levetiracetam, perampanel, and zonisamide. Cannabidiol, currently under review by the FDA, may receive approval for the treatment of LGS and Dravet syndrome this year; based on this, it is likely to be included in Tier 1 or 2 of the LGS treatment algorithm. AEDs categorized into Tier 3 were potentially valuable therapies that lack systematic trial data to support their efficacy, and include felbamate, lacosamide, cenobamate, and brivaracetam. A category of AEDs to be avoided in the treatment of LGS was determined during discussions and included treatments that are used less frequently for LGS, plus treatments that have the potential to exacerbate certain seizure types in some patients. Treatments in this category included carbamazepine, eslicarbazepine, gabapentin, phenytoin, pregabalin, oxcarbazepine, and vigabatrin. Therapies considered complementary to AED treatment were also assessed and included nonpharmacologic interventions, community services, and psychosocial treatments. The proposed treatment algorithm is presented in Figure 1. Conclusions: Effective and appropriate medications are recommended for the treatment of LGS in adult patients, as outlined in Tiers 1 and 2 of the proposed treatment algorithm. Tier 3 AEDs require further trial data to support their efficacy. A group of AEDs to be avoided in the treatment of LGS has also been identified. Funding: The advisory board was convened and funded by Eisai Inc. All authors were participants of the advisory board and were responsible for the decision to develop and submit this abstract. Medical writing support, under the direction of the authors, was funded by Eisai Inc., in accordance with GPP3 guidelines.