Exploration of the Potential for Plasma Protein Binding Displacement and Drug-Drug Interactions of Valproate in Combination with Cannabidiol
Abstract number :
1.453
Submission category :
7. Antiepileptic Drugs / 7E. Other
Year :
2018
Submission ID :
540271
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Bola Tayo, GW Research Ltd; Elinor Ben-Menachem, University of Gothenburg; Boudewijn Gunning, Stichting Epilepsie Instellingen Nederland; Carmen Maria Arenas Cabrera, Hospital Virgen del Rocío; Julie Crockett, GW Research Ltd; Lesley Taylor, GW Resea
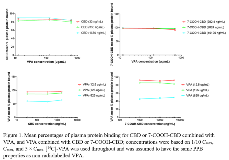
Rationale: A pharmaceutical formulation of highly purified cannabidiol (Epidiolex®; CBD) has been shown to be effective in treating seizures associated with Lennox-Gastaut and Dravet syndromes. Many patients with epilepsy use multiple antiepileptic drugs, including valproate (VPA). As CBD and VPA are known to bind extensively to plasma proteins, an in vitro study explored the potential for plasma protein binding (PPB) displacement of CBD, its major circulating metabolite 7-carboxy-CBD (7-COOH-CBD), and VPA when combined in human plasma. Additionally, as in vitro data indicated CBD may interact with multiple cytochrome P450 and uridine 5’-diphospho-glucuronosyltransferase enzymes, a phase 2 trial in adult patients with epilepsy investigated any steady-state pharmacokinetic (PK) drug-drug interactions (DDI) between GW’s formulation of CBD and VPA, and its metabolite 2-propyl-4-pentenoic acid (4-ene-VPA). Methods: Human plasma pooled from 3 healthy donors was used to assess the PPB displacement of (i) CBD in the presence of VPA, (ii) 7-COOH-CBD in the presence of VPA, (iii) VPA in the presence of CBD, and (iv) VPA in the presence of 7-COOH-CBD. Plasma concentrations of total VPA and 4-ene-VPA in patients with epilepsy were determined using validated bioanalytical methods. To assess the effect of CBD or placebo (10 days of titration followed by 10 mg/kg twice daily for 14 days) on VPA and 4-ene-VPA plasma exposures, a standard 90% confidence interval approach using a linear mixed-effect model was used to derive the treatment ratios for Cmax and AUCtau with and without CBD. Patients received their maintenance dose of VPA (median of 500 mg twice daily). Results: CBD was 86–90% bound and remained 80–91% bound in the presence of [14C]-VPA, independent of concentration. Similarly, 7-COOH-CBD was 98–99% bound and remained 93–99% bound in the presence of [14C]-VPA, independent of concentration. [14C]-VPA was 52–93% bound and although concentration-dependent, was unaffected in the presence of CBD and 7-COOH-CBD (Figure 1). Twenty patients with epilepsy on stable doses of VPA were randomized to treatment (16 CBD; 4 placebo). Two CBD-treated patients withdrew early; 1 due to hypertransaminasemia (> 3.0 × upper limit of normal) and 1 due to diarrhea and nausea. Following multiple days of twice daily dosing of VPA+CBD (n=10), there were minor decreases in plasma Cmax and AUCtau of VPA (≤ 16%) and 4-ene-VPA (≤ 30%) on Day 26 (VPA+CBD) compared with Day 1 (VPA alone); ratio point estimates were similar for placebo patients (n=3) (Table 1). Adverse events (AEs) occurred in 14 (88%) patients taking CBD and 1 (25%) taking placebo, all of which were mild or moderate in severity. The most common AE was diarrhea in 11 (69%) CBD-treated patients. Conclusions: Combining VPA with CBD or 7-COOH-CBD did not alter the PPB parameters at clinically relevant concentrations, suggesting that VPA in combination with CBD is not likely to increase the free drug fraction of either drug in human plasma. Furthermore, co-administration of CBD with VPA showed no evidence of any effect on the PK of VPA or 4-ene-VPA that would be considered clinically relevant. The safety results were consistent with the safety profile of CBD. Funding: GW Research Ltd, Cambridge, UK
.tmb-.png?Culture=en&sfvrsn=d8f530fe_0)