Finding treatment for NEonatal seizures using Medication Off-patent (NEMO): an phase I/II dose finding and feasibility trial of bumetanide for second line treatment of neonatal seizures
Abstract number :
3.339
Submission category :
Late Breakers
Year :
2013
Submission ID :
1867453
Source :
www.aesnet.org
Presentation date :
12/7/2013 12:00:00 AM
Published date :
Dec 5, 2013, 06:00 AM
Authors :
R. M. Pressler, G. B. Boylan, L. de Vries, N. Marlow, M. Blennow, S. Vanhatalo, G. Pons, S. Zohar, V. Jullien, B. Hallberg, D. Murray, M. Toet, B. Murphy, M. Levene, R. Swarte, L. Hellstr m-Westas, C. Chiron, J. Rennie, B. Mangum, J. Cross
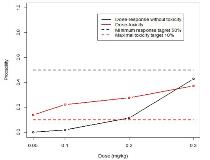
Rationale: Preclinical data have suggested that bumetanide may be an effective treatment for neonatal seizures by blocking the neuronal NKCC1 co-transporter, reducing intracellular chloride concentration and consequently reducing or reversing the depolarizing action of GABA in the immature brain. We aimed to assess the safety and optimal dose of bumetanide for the treatment of neonatal seizures in a phase I/II dose finding and feasibility clinical trial.Methods: Using a bivariate Bayesian sequential dose-escalation design, we performed an open label study to evaluate the dose efficacy and dose-toxicity relationship as well as pharmacokinetics of iv bumetanide given in 4 doses over 36 hrs. Full term babies aged <48 h with hypoxic ischaemic encephalopathy and seizures not responding to phenobarbitone were allocated via bivariate continual reassessment method to one of the 4 dose levels (0.05-0.3mg/kg). Adverse events and seizure burden were assessed continuously, the latter with continuous EEG monitoring. Pharmacokinetic data were evaluated using a population approach.Results: Of 30 screened babies meeting eligibility criteria, fourteen babies (10 male) were included (4 at dose 0.05 mg/kg, 3 at 0.1 mg/kg, 6 at 0.2 mg/kg and 1 at 0.3 mg/kg). Nine neonates completed treatment as per protocol, 3 were withdrawn for clinical reasons and 1 due to an adverse reaction (moderate dehydration). All but 1 baby also received aminoglycosides. No short term, dose-limiting toxicity was observed but 3/14 babies were found to have hearing loss on later auditory testing (delayed toxicity). EEG analysis showed that 5/14 babies had reduction in seizure burden (>80%) at 3-4 h after the initial dose (efficacy as per protocol). After taking delayed toxicities into account, the posterior estimated dose-response relationships at the end of the trial indicate that all doses are estimated to be toxic (Fig 1). Subsequent data analysis showed that 12/14 babies required additional rescue antiepileptic drugs for seizure control and seizure control was sustained in only 2/14 babies. The final PK model was a 2-compartment model with zero-order infusion and first-order elimination; clearance was 0.327 ml/min/kg, steady-state volume was 0.229 l/kg, and elimination half-life 8.43 h. There was no significant relationship between bumetanide exposure and hearing loss; however, the reliability of this result is questionable because of the small number of included neonates. Due to the adverse dose efficacy and dose-toxicity relationship, the trial was terminated prematurely.Conclusions: Although bumetanide demonstrated good short-term tolerability, we found a much higher incidence of hearing loss compared to the literature or our own historical data. This may be explained by accumulation of risk factors (hypoxia, aminoglycoside, loop diuretic) in an increased susceptibility to drug-induced hearing loss in the immature brain. Due to adverse benefit-risk ratio, further efficacy trials using bumetanide at this dose regimen cannot be recommended.