Hyperactive mTOR Signaling Results in Sex- and Age-Specific Differences in Early Life Communication
Abstract number :
1.061
Submission category :
1. Basic Mechanisms / 1E. Models
Year :
2018
Submission ID :
500500
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Matthew Binder, Baylor University; Samantha Hodges, Baylor University; Suzanne Nolan, Baylor University; Andy Holley, Baylor University; and Joaquin Lugo, Baylor University
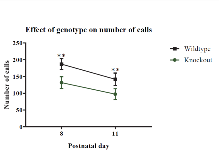
Rationale: A signaling cascade that plays a crucial role in the development of an epileptic phenotype is the PI3K/AKT/mTOR pathway. Mouse models that illustrate this connection include Tsc1 or Tsc2 heterogenous mice and neuron subset-specific (NS)-Pten knockout mice. While numerous studies have investigated ultrasonic vocalizations (USVs) in Tsc1 and Tsc2 heterogenous mice, none have investigated USVs in NS-Pten knockout mice using the Avisoft recording system. In addition to USVs being irregular in mouse models of epilepsy, these vocalizations are also dysregulated in the highly comorbid autism spectrum disorder. Therefore, the further elucidation of pup vocalizations in the NS-Pten model, a model of both autism and epilepsy, may clarify not only the underlying role of mTOR in an epileptic phenotype, but also mTOR’s role in epilepsy’s comorbidity with autism. Methods: The present study utilized a maternal separation paradigm in order to characterize neonatal USVs in NS-Pten wild-type (WT) and knockout (KO) males and females (male WT n = 14, male KO n = 12, female WT n =15, female KO n =15). On postnatal days (PD) 8 and 11, the pups were individually removed from their mother and placed into a sound attenuated chamber. An Avisoft broad spectrum microphone then recorded the isolation- induced pup USVs for a 2 minute duration. Results: We found that knockout pups emitted fewer vocalizations across both sexes (F(1,50) = 7.06, p = .01), and males emitted fewer vocalizations than females (F(1,50) = 6.96, p = .01). KO males had calls of a shorter duration (t(3588) = 5.08, p < .001) and lower peak amplitude (U = 1217712, p < .001) on day 8, while showing a shorter duration (U = 625954, p < .001), lower peak amplitude (U = 676598, p < .001), and higher peak frequency (t(2397) = 9.33, p < .001) and fundamental frequency on day 11 (U = 658154, p < .001). KO females vocalized at a lower peak amplitude (U = 2386907, p < .001) and lower fundamental frequency (U = 2948392, p = .04), and a higher peak frequency on day 8 (U = 2853297, p < .001), while showing a shorter duration (U = 1487283, p < .001) and higher peak frequency (U = 1161870, p < .001) and fundamental frequency on day 11 (U = 1305243, p < .001). Spectrographic analyses also revealed significant differences in call type on PD 8 and PD 11 for each genotype (X2(7, N = 8718) = 445.83, p < .001),(X2(8, N = 6549) = 101.43, p < .001). On PD 8 knockout animals emitted significantly more frequency steps and upward calls, while emitting fewer complex, two syllable, composite and chevron calls than wild-type mice (p < .05). On PD 11, KO animals emitted more frequency steps and upward calls and fewer complex and two syllable calls (p < .05). There were also sex-specific call type differences on PD 8 and PD 11(X2(7, N = 8718) = 61.58, p < .001),(X2(8, N = 6549) = 53.46, p < .001). On PD 8, Males emitted significantly more two syllable cries, whereas females produced more frequency steps calls (p < .05). On PD 11, males emitted more two syllable, short, and downward call types, while females produced significantly more chevron and frequency steps calls (p < .05). Conclusions: These findings demonstrate that deletion of NS-Pten results in significant decreases in vocalizations across both sexes at each time point assessed. Additionally, our findings indicate that the aberrant USVs and increased call duration seen in other mTOR models are also present in NS-Pten KO mice. Our study provides evidence of a connection between hyperactive mTOR signaling and irregular ultrasonic vocalizations. Furthermore, since NS-Pten knockout mice serve as both an epilepsy and autism model, our results may provide new insights into the relationship between the two conditions. Funding: This work was supported by NIH Grant: R15S088776
.tmb-.png?Culture=en&sfvrsn=fe091818_0)