In Vivo High-Resolution DTI Demonstrates Excellent Diagnostic Accuracy and Subfield-Specific Diffusion Abnormalities in Temporal Lobe Epilepsy With Hippocampal Sclerosis
Abstract number :
2.167
Submission category :
5. Neuro Imaging / 5A. Structural Imaging
Year :
2018
Submission ID :
501191
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Donald W. Gross, University of Alberta; Sarah Treit, University of Alberta; Graham Little, University of Alberta; Trevor A. Steve, University of Alberta; Laura M. Schmitt, University of Alberta; Tomasz A. Nowacki, University of Alberta; and Christian Beau
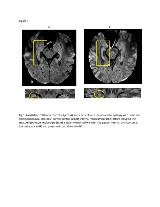
Rationale: Previous Diffusion Tensor Imaging (DTI) studies have demonstrated increased hippocampal mean diffusivity (MD) in TLE with Hippocampal Sclerosis (HS)[1, 2]. Given the small volume of the hippocampus relative to the resolution of conventional brain DTI (around 8mm3), partial volume averaging is however a significant confounding variable. Recent studies have demonstrated that hippocampal subfield pathology can predict surgical outcome leading to a growing interest in studying hippocampal subfields in vivo with MRI[3]. While there is interest in studying diffusion properties of hippocampal subfields, the spatial resolution of conventional DTI limits this possibility. We have developed a high-resolution (1x1x1mm3) hippocampal DTI acquisition sequence providing excellent SNR in a clinically feasible scan time[4]. The purpose of this study was to assess hippocampal and subfield diffusion properties of TLE patients in order to determine whether high-resolution DTI can provide clinically relevant information. Methods: Nineteen control subjects and 18 TLE patients (7 TLE+ unilateral HS, 3 TLE+ bilateral HS, 8 TLE without HS) were scanned on a 3T Siemens Prisma including 0.9mm isotropic MPRAGE, quantitative T2 and DTI (single shot 2D GRAPPA, TE=72ms, TR=2800 ms, 10 averages, 10 gradient directions at b=500 s/mm2 and 10 b0s, scan time 5:18). Whole hippocampal volumes, T2 and MD were compared between groups. Blinded review of mean Diffusion Weighted Images (DWI) was performed to assess internal hippocampal architecture. MD maps were evaluated qualitatively to assess lateral–mesial and caudal–rostral variability as well as correlation between MD and histology for three subjects who have undergone surgery. Results: HS was associated with significant reduction in hippocampal volume, elevation in T2 and elevation in MD. Internal hippocampal architecture and the stratum lacunosum moleculare (SLM) were clearly visualized in controls, TLE without HS and in the contralateral hippocampus of unilateral HS subjects on DWI but poorly visualized in TLE patients with HS (Fig 1). Blinded review of DWI for the presence of internal hippocampal architecture demonstrated sensitivity of 83% and specificity of 97% in detecting HS. MD maps for TLE with HS subjects demonstrated dramatic variability regarding the lateral–mesial and caudal–rostral abnormalities (Fig 2). For three subjects (one patient with a ganglioglioma and a normal hippocampus, one patient with Type 2 HS and one with Type 3 HS), hippocampal subfield-specific MD abnormalities correlated well with histological findings (subfield-specific neuronal loss and gliosis) (Fig 2). Conclusions: High resolution DTI obtained in a clinically feasible scan time demonstrated high sensitivity and specificity in detecting HS in TLE patients. The observed lateral–mesial and caudal–rostral MD heterogeneity is consistent with pathological literature[3, 5]. The excellent correlation between hippocampal subfield MD findings and histology for three subjects suggests that high-resolution DTI has the potential to predict HS subtype in vivo which would result in improved ability to predict surgical outcomes preoperatively.References:1. Salmenpera TM, et al. Epilepsy Res. 2006;71(2-3):102-106.2. Bernhard BC, et al. Ann Neurol. 2016;80(1):142-153.3. Blumcke I, et al. Acta Neuropathol. 2007;113(3):235-244.4. Treit S., et al. Neuroimage. 2018.5. Thom M, et al. Epilepsy Res. 2012;102(1-2):45-59. Funding: This work has been supported by an operating grant from the Canadian Institute of Health Research.
.tmb-.png?Culture=en&sfvrsn=d433353a_0)