IV Carbamazepine as Short-Term Replacement Therapy for Oral Carbamazepine in Adults with Epilepsy: Primary Results of a Phase III Safety Trial
Abstract number :
3.301
Submission category :
Late Breakers
Year :
2013
Submission ID :
1860085
Source :
www.aesnet.org
Presentation date :
12/7/2013 12:00:00 AM
Published date :
Dec 5, 2013, 06:00 AM
Authors :
S. Dheerendra, P. Klein, V. Biton, L. Zhang, U. Kalu, D. Lee, J. Halford
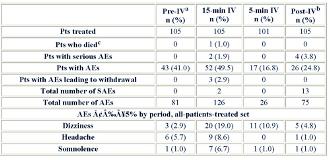
Rationale: An IV formulation of carbamazepine (CBZ) solubilized in a cyclodextrin matrix has been developed. A previous study of adults with epilepsy demonstrated that IV CBZ infused over 15 or 30 minutes every 6 hours at a 70% dosage conversion from patients oral dosages provided comparable exposures to oral CBZ.1 In this Phase III study, we assessed the safety and tolerability of IV CBZ administered as multiple, 15-minute infusions to adults with epilepsy on stable dosages of oral CBZ, and as a single, 5-minute infusion.Methods: Adults with epilepsy receiving oral CBZ (1,200 2,000 mg/day) enrolled in an open-label study. A 27-day period of stable oral CBZ dosing was followed by a 7-day/6-night confinement with IV CBZ administration (and no oral CBZ), and, finally, a 28-day follow up after last dose of IV CBZ (during which dosages of oral CBZ were resumed). Patients compliance with their oral CBZ regimens was evaluated on Day 14. The confinement began on Day 1. Patients received their last doses of oral CBZ on Day 0. From Day 1 until the morning of Day 4, IV CBZ was administered at 70% of daily oral dosages at 6-hour intervals. Oral CBZ regimen was resumed 6 hours after last IV dose. On Day 5, patients were discharged 2 hours (those receiving immediate-release formulation) or 4 hours (those receiving extended-release formulation) following the first re-administration of oral CBZ. Follow up comprised the completion visit on Day 18 and a telephone interview on Day 32. Safety assessments included periodic physical examinations, laboratory evaluations, ECGs, and adverse event (AE) data collection.Results: 153 patients were screened at 23 US sites: 108 enrolled, 105 were treated, and 101 completed the study. Four patients withdrew (3 because of AEs and 1 withdrew consent). Baseline demographics for the 105 treated patients were mean age: 41 years (range: 18 76 years); 51% male; mean epilepsy duration: 25 years; and mean number of seizures/week: 1.4 (range: 0 20). Each patient underwent 12 protocol-defined, 15-minute infusions and 1 protocol-defined 5-minute infusion (table). During the pre-IV period, 41% of patients had 81 AEs. None were SAEs or led to withdrawal. During the 15-minute IV period, 50% had 126 AEs. Two patients had SAEs (cerebral hemorrhage and convulsions), and 3 had AEs that led to withdrawal. During the 5-minute IV period, 17% of patients had 26 AEs. None were SAEs or led to withdrawal. During the post-IV period, 25% of patients had 75 AEs, 4 had 13 SAEs, and no patients withdrew because of AEs. No treatment-related significant abnormalities were observed in the clinical and laboratory safety data or ECGs.Conclusions: IV CBZ administered as multiple, 15-minute infusions was safe and well-tolerated as short-term replacement in adults with epilepsy on stable dosages of oral CBZ. IV CBZ as a single, 5-minute infusion also appeared to be safe and well-tolerated. 1Walzer MA, et al. Epilepsy Curr. 2011;11(1 Suppl 1):Abstract #1.259.