Long-Term Retention on Adjunctive Brivaracetam in Adults With Focal Seizures Previously Exposed to Carbamazepine, Lamotrigine, Levetiracetam, or Topiramate: A Post hoc Analysis
Abstract number :
1.294
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
498031
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Melinda Martin, UCB Pharma; Svetlana Dimova, UCB Pharma; Sami Elmoufti, UCB Pharma; Cédric Laloyaux, UCB Pharma; and Steve S. Chung, Banner University Medical Center - Phoenix
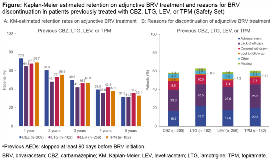
Rationale: A post hoc analysis of three 12-week, placebo (PBO)-controlled trials of adjunctive brivaracetam (BRV) in adults with focal seizures demonstrated that BRV has a similar and significant efficacy over PBO regardless of previous treatment failure to carbamazepine (CBZ), lamotrigine (LTG), levetiracetam (LEV), or topiramate (TPM) (Asadi-Pooya AA, et al. Epilepsy Res 2017;131:70–5). The aim of this post hoc analysis was to explore the long-term effectiveness of adjunctive BRV in adults with focal seizures previously treated with CBZ, LTG, LEV, or TPM. Methods: This was a post hoc analysis of a double-blind, PBO-controlled trial (N01358; NCT01261325) and corresponding open-label extension (N01379; NCT01339559; cut-off 15 March 2017) of adjunctive BRV in adults with focal seizures. Analysis was performed in patients randomized to BRV 100 or 200 mg/day (pooled) in the initial double-blind trial. Retention rates from the first BRV dose in the double-blind trial (Kaplan-Meier [KM] analysis) and reasons for BRV discontinuation were assessed in subgroups of patients who had tried CBZ, LTG, LEV, or TPM and stopped at least 90 days prior to BRV initiation. Results: 503 patients were randomized to BRV 100 or 200 mg/day. In the overall population, the KM-estimated retention on BRV was 71.0%, 59.6%, 50.9%, 40.9%, and 32.4% at 1, 2, 3, 4, and 5 years. 296 (58.8%) patients discontinued BRV by the time of data collection (median time to discontinuation 370 days). Most common reasons for discontinuation were lack of efficacy (19.3%) and adverse event (16.1%). The baseline demographics and epilepsy characteristics in the subgroups of patients previously treated with CBZ (n=209), LTG (n=162), LEV (n=256), or TPM (n=182) were generally similar (Table). Patients had long epilepsy duration, high baseline focal seizure frequency, and high number of prior AEDs. The KM-estimated retention rates on adjunctive BRV were generally similar across the subgroups with previous CBZ, LTG, LEV, or TPM exposure, ranging from 64.8%–73.2% at 1 year, 48.1%–60.3% at 2 years, and 41.9%–49.9% at 3 years (Fig A). Similar proportion of patients discontinued BRV irrespective of the previous AED subgroup (CBZ 58.4%; LTG 63.0%; LEV 58.6%; TPM 60.4%). Reasons for BRV discontinuation were also similar with 23.0%–25.3% discontinuing due to lack of efficacy and 16.7%–22.2% due to adverse event (Fig B). The median time to discontinuation was 379 days in the CBZ subgroup, 298 days in the LTG subgroup, 315 days in the LEV subgroup, and 340 days in the TPM subgroup. Conclusions: This post hoc analysis demonstrated similar long-term retention rates on adjunctive BRV and similar reasons for BRV discontinuation in adults with focal seizures previously treated with CBZ, LTG, LEV, or TPM. Adjunctive BRV provides long-term effectiveness in patients who failed common AED treatments, including LEV. This analysis supports that common AED treatment failures, including LEV, should not preclude the use of BRV to treat patients with focal seizures. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=be7af795_0)