Long-Term Safety and Efficacy of Add-on Cannabidiol (CBD) Treatment in Patients with Dravet Syndrome (DS) in an Open-Label Extension (OLE) Trial (GWPCARE5)
Abstract number :
1.470
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
550389
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Ingrid E. Scheffer, University of Melbourne; Jonathan Halford, Medical University of South Carolina; Rima Nabbout, Hôpital Universitaire Necker - Enfants Malades, Service de Neurologie Pédiatrique Centre de Référence Épilepsies Ra
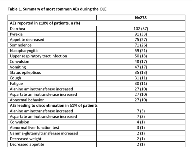
Rationale: DS is an intractable epilepsy, with onset typically in the first year of life and a high mortality rate. Efficacy and safety of GW Pharmaceuticals’ formulation of CBD for seizures associated with DS have been demonstrated in a randomized, placebo-controlled trial, GWPCARE1 (NCT02091206), with results pending from a second ongoing trial GWPCARE2 (NCT02224703). Here, we report long-term safety and efficacy results of the second interim analysis of the OLE (NCT02224573) of these studies. Methods: Patients who completed GWPCARE1/2 could enroll in GWPCARE5 and receive a plant-derived pharmaceutical formulation of highly purified CBD (100 mg/mL) in oral solution. Initially, dose was titrated up to the target 20 mg/kg/d given as 2 doses, which could be decreased or increased to a maximum of 30 mg/kg/d at the investigator’s discretion. The primary endpoint was safety. Secondary endpoints were change from baseline in convulsive and total seizure frequency every 12 wks and Subject/Caregiver Global Impression of Change (S/CGIC). Results: Of 289 patients with DS who completed the randomized trials (RTs), 278 enrolled in the OLE study. The median treatment time (range) was 50 wks (>1–99 wks). Overall, 14% of patients had completed, 52% were still ongoing, and 34% had withdrawn. Mean age of the patients was 9.8 yrs, 97% were < 18 yrs, and 50% were male. Patients were taking a median of 3 concurrent AEDs with 68% taking clobazam, 63% valproate, and 39% stiripentol. At baseline of the RTs, patients had median 12 convulsive and 32 total seizures per 28 d. The mean (SD) of patients’ modal dose of CBD during the OLE was 22 (5). Adverse events (AEs) were reported by 96% of patients, serious AEs by 32%, and 7% discontinued due to AEs (see Table 1 for most common AEs). Twenty-six patients (9%) had elevated liver transaminase > 3× the upper limit of normal; 23/26 (88%) were on concomitant valproate; none met Hy’s law criteria for severe liver injury. The 2 deaths reported during the study were not attributed to CBD treatment by the investigator. Median % reductions from baseline, assessed every 12 wks, ranged from 44%–57% for convulsive and 49%–67% for total seizures and were consistent over 72 wks (Table 2). Last observation carried forward analysis showed similar results. Most patients/caregivers (82% at 24 wks; 87% at 48 wks) reported improvements in patient’s overall condition on the S/CGIC. Conclusions: Long-term, add-on CBD treatment had a similar safety profile to that observed in the RTs. Sustained reductions in convulsive and total seizures were observed up to 72 wks, with the majority of patients/caregivers reporting an improvement in overall condition. Results of this OLE demonstrate the long-term benefits of CBD treatment for patients with DS. Funding: GW Research Ltd, Cambridge, UK
.tmb-.png?Culture=en&sfvrsn=aea151ea_0)