Low-Grade Inflammation in the Epileptic Hippocampus Contrasts With Explosive Inflammation Occurring in the Acute Phase Following Epileptogenic Brain Insults
Abstract number :
3.107
Submission category :
2. Translational Research / 2D. Models
Year :
2018
Submission ID :
506220
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Nadia Gasmi, Lyon Neuroscience Research Center, Epilepsy Institute; Amor Belmeguenaï, Lyon Neuroscience Research Center, Epilepsy Institute; Michaël Ogier, IRBA; Béatrice Georges, Lyon Neuroscience Research Center, Epilepsy Institute; Sandr
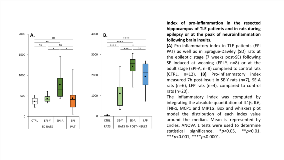
Rationale: Accumulating evidence from studies performed on human hippocampi and in animal models of temporal lobe epilepsy (TLE) supports a role for neuroinflammation as a primary driver of epileptogenesis occurring after brain insults and as a self-perpetuating factor of epileptic seizure activity. However, to date, a thorough knowledge into how the hippocampal inflammatory status differs between epileptic patients on one hand, and between epilepsy and epileptogenesis on the other hand, is still lacking. Therefore, we established the calculation of an index of inflammation integrating the absolute quantitation of prototypical inflammatory gene transcripts in the resected hippocampus of TLE patients and in rats during the chronic phase of epilepsy following status epilepticus (SE) or at the peak of neuroinflammation after brain insults. Methods: Hippocampal tissue obtained at surgery from 22 patients with medically intractable TLE were flash-frozen in liquid nitrogen and stored at -80°C. In animal studies, rats were subjected to pilocarpine-induced SE either at weaning (SE-Y, n=7) or at the young adult stage (42 days; SE-A, n=6) or to biLateral Fluid Percussion at 9-10 weeks (LFP, n=4). Hippocampal tissue was removed either at the apparent peak of inflammation (7 hrs post-insult: SE-Y, SE-A, LFP) or 7 weeks after epilepsy onset when rats were subjected to SE (EPI-Y and EPI-A). Transcripts levels of pro- and anti-inflammatory cytokines, chemokines and markers of inflammatory cells were quantitated using real-time PCR. Three indexes of inflammation were calculated by integrating the absolute quantitation of 1. pro-inflammatory markers (IL1b, IL6, TNFa, MCP1, MIP1a); 2. anti-inflammatory markers (IL4, IL10, IL13); 3. Cell markers (CD11b for tissue microglia/macrophages, GFAP for astrocytes). Results: We report a very high variability of the inflammation index in TLE patients (mean ± SD: 441 ± 288), some with values close to the limit of detection while others had much higher values. Similarly, the modeling carried out in rats having developed epilepsy after SE induced either at weaning (EPI-Y) or in young adults (EPI-A) shows in the first case, that the inflammation index is indiscernible from that of the controls, while in EPI-A the inflammation index is higher (p < 0.01). In addition, inflammation in the epileptic rats (EPI-A) has no common measure with the explosive inflammation observed after moderate (LFP) to severe (SE) cerebral aggression (p < 0.0001), whether this is followed by a real epileptogenesis (SE) or not (LFP). Conclusions: Our study provides strong evidence that: 1. some, not all, TLE patients may present with a hippocampal inflammatory status that is likely to correspond to low-grade inflammation, suggesting that neuroinflammation by itself cannot explain ictogenesis in epilepsy; 2. the explosive neuroinflammation that occurs early after a brain insult may be important, but not sufficient, to trigger epileptogenesis. Funding: This work was supported by a grant from the Direction Générale de l’Armement (French Ministry of Defense).