Neuromagnetic Dynamics of Working Memory in Temporal Lobe Epilepsy and in Healthy Controls
Abstract number :
3.465
Submission category :
3. Neurophysiology / 3D. MEG
Year :
2018
Submission ID :
555718
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Bradley Albritton, Louisiana Tech University; Sangeeta Nair; Diana Pizarro, University of Alabama at Birmingham; Jeff Killen, University of Alabama at Birmingham; Sandipan Bankim Behari Pati; Roy C. Martin, University of Alabama at Birmingham; Jerzy P. Sz
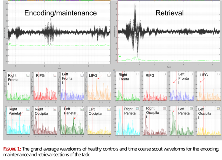
Rationale: Working memory (WM) describes a cognitive system which allows for the transient storage and utilization of information. Working memory describes multiple integrated processes. A stimulus is first encoded into working memory, stored and refreshed through rehearsal or maintenance of the memory trace, and ultimately utilized or retrieved to perform a goal-oriented action. Several functional MRI and PET studies highlighted a group of brain regions as critical to working memory including the dorsolateral prefrontal cortex (DLPFC), parietal and occipital regions, and the left supramarginal gyrus. Very few studies utilized neurophysiologic signals (MEG/EEG) to study sequential activation during WM tasks and some findings in the MEG/EEG working memory literature have been variable, although generally implicating theta activity to play a major role in working memory functions, in particular during encoding. There are no MEG data describing network working memory changes in patients with epilepsy. Methods: We recorded MEG data on 5 adult patients with temporal lobe epilepsy (two right, three left) and six healthy controls while performing a Sternberg working memory task using a 148-channel neuromagnetometer system, housed inside a magnetically- shielded room. In the Sternberg task, each trial comprised four phases, (1) a fixation phase which lasted 1.0 sec and functioned as the baseline; (2) an encoding phase which lasted 2.0 sec and consisted of 1, 3, 5 or 7 letters being presented simultaneously, (3) a maintenance phase that jittered 2-2.5 seconds where the six letters disappeared from the grid, and (4) a retrieval phase which lasted 4 sec and required participants to respond as to whether the single letter probe was included in the original encoding set. Each participant's MEG data were co-registered with structural T1-weighted MRI data prior to source space analysis. Cortical networks were imaged through a linearly constrained minimum variance vector (LCMV) beamformer which employs spatial filters in the frequency domain to calculate source power for the entire brain volume at different frequency bands (4-8 Hz, 8-13 Hz, 13-25 Hz and 25-50Hz). (http://neuroimage.usc.edu/brainstorm). Group images depicting sequential oscillatory changes were created during encoding, maintenance and retrieval in each frequency band. Results: Encoding, maintenance and retrieval activated bilateral network involving frontal, parietal and occipital regions in both patients and healthy subjects. Robust theta oscillatory response was seen during the maintenance/rehearsal phase once the letters disappeared (figure 1). The most robust activation is in the left inferior frontal gyrus in healthy subjects (figure 2a). Figure 2b shows oscillatory theta changes in healthy subjects during retrieval, particularly over the right supramarginal gyrus. Patients showed tendency to recruitment of additional frontal regions. There was no clear effect of the side of epilepsy likely due to small sample size. Conclusions: Our study provided evidence for the role of theta oscillations during Sternberg working memory tasks. These oscillatory responses mediate the maintenance/rehearsal phase and are most prominent after the stimulus disappear. It also provides preliminary evidence of patterns of reorganization of working memory networks in temporal lobe epilepsy. Funding: This data and research was partially funded through EPSCoR NeuroNEM as well as GRSP AL EPSCoR grants.
.tmb-.png?Culture=en&sfvrsn=af6d1044_0)