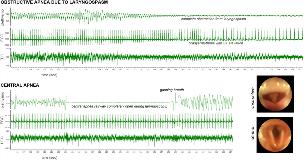
OBSTRUCTIVE APNEA DUE TO LARYNGOSPASM DURING SEIZURES, BUT NOT CENTRAL APNEA, CAUSES HYPOXIC CARDIAC DERANGEMENTS IN RATS
Abstract number :
2.375
Submission category :
Year :
2014
Submission ID :
1868927
Source :
www.aesnet.org
Presentation date :
12/6/2014 12:00:00 AM
Published date :
Dec 4, 2014, 06:00 AM
Authors :
Mark Stewart, Ko Nakase, Krishnamurthi Sundaram, Joshua Silverman and Richard Kollmar
Rationale: Sudden Unexplained Death in Epilepsy accounts for up to 17% of deaths in epileptic persons. Seizure spread into subcortical regions, including hypothalamus and medulla, generates autonomic nervous system derangements that can cause significant cardiovascular and respiratory dysfunction that are almost certainly the link between seizure activity and death. With our rat model, which uses kainic acid as a convulsant in rats anesthetized with urethane, we are able to monitor invasively and non-invasively systemic physiology during seizures. Methods: Male Sprague Dawley rats(180-340 g) were anesthetized with urethane (1.5 g/kg ip) and studied in a plethysmogragh with simultaneous continuous recordings of ECG (limb leads), EEG (screw electrodes over dorsal hippocampus), and video laryngoscopy. Animals received kainic acid (KA; 10-12 mg/kg ip) for seizure induction. Tidal-breathing was studied with head-out plethysmography. Flow-volume loops and associated parameters (breathing rate, tidal volume, peak expiratory and inspiratory flow, etc.) were derived from the flow rates. All animals received KA. Sub-groups received: 1) no additional treatment; 2) endotracheal intubation before KA; 3) bilateral superior laryngeal nerve transection to prevent reflex laryngospasm; or 4) systemic atropine to minimize salivation (2 mg/kg ip). Results: Periods of central apnea, determined by abrupt cessation of breathing at the end of expiration for periods up to 20 seconds and video evidence of an open airway, occurred in all groups, including the intubated group (13 rats total; KA-only: 2/7 rats had 2-6 apneic periods/rat; intubated: 3/6 rats had 1-3 apneic periods/rat; SLN lesion: 6/6 rats had 1-13 apneic periods/rat; atropine: 3/6 rats had 1-2 apneic periods/rat). Obstructive apnea, determined by progressive decreases in tidal volume until flow stopped and video evidence of a closed glottis, was observed in 8 rats (2 from the KA-only group, and all 6 of the SLN lesioned rats. In seven of these animals, there was laryngoscopic evidence of complete glottic closure. All of the obstructive apneic episodes were associated with hypoxic cardiac derangements as evidenced by ST segment elevation and progressive bradyarrhythmia, ending in death in 7/8 animals. In one animal, breathing restarted in the form of gasping breaths and recovery of heart rate. None of the central apnea periods was associated with cardiac derangement. Conclusions: Seizures produce periods of central and obstructive apnea in our rat model. Seizure-induced laryngospasm resulted in obstructive apnea with rapid, hypoxic changes in the ECG, but central apneic periods did not. We conclude that severe laryngospasm represents a likely contributor to the seizure and hypoxemia-induced conditions that result in death.