Optimization of Channel Structure and Operating Condition of Neuroprotective Focal Brain Cooling Device
Abstract number :
1.223
Submission category :
4. Clinical Epilepsy / 4C. Clinical Treatments
Year :
2018
Submission ID :
498570
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Takuto Abe, Kyoto University; Takao Inoue, Yamaguchi University Graduate School of Medicine; Koichi Fujiwara, Kyoto University; Sadahiro Nomura, Yamaguchi University Graduate School of Medicine; Hirochika Imoto, Yamaguchi University Graduate School of Med
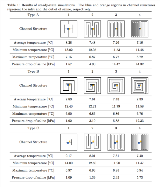
Rationale: Therapeutic hypothermia has been drawing an attention as a potential treatment for patients in the acute phase of stroke and traumatic brain injury although it has side effects. In such disease, epileptiform discharges occur along the border of the injured area. Moreover, the prolonged excessive neuronal activities possibly result in a change of the injured site to epileptogenicity. Focal brain cooling (FBC) can exert a neuroprotective effect more effectively than the therapeutic hypothermia because some experiments with rats showed that epileptiform discharges induced by potent convulsant agents or artificial cerebral infarction were suppressed by cooling the brain surface to very low temperature of 25-10 ? (J Neurosurg 2006;104:150-156, Epilepsia 2012;53(3):485-493, Brain Res 2012;1497:53-60). However, in the case of the human brain, it is more difficult to lower focal cerebral temperature than the animal’s brain because of the large brain volume. Thus, we aim to realize an FBC system with cooling water circulation. Specifically, we develop a cooling device made of titanium with a water channel inside, implant on the patient’s dura mater, and let saline flow in the device to remove heat from the brain surface. In this research, we attempted to optimize the channel structure of the cooling device and flow rate of saline so that both the highest cooling performance and the lowest pressure drop were achieved simultaneously. Methods: The head model approximated the upper half of the head with a hemisphere and consisted of a brain, cerebrospinal fluid, dura matter, skull, soft tissue, and the cooling device. The heat transfer in the cooling device, saline, and biological tissue and mass transfer of saline were calculated by computational fluid dynamics (CFD). We compared three types of basic channel structures of the cooling device: serpentine, spiral, and branched structures. For each basic channel structure, we created four structures by changing the channel width (complexity). First, a steady-state analysis was performed for all 12 device models to confirm cooling performance and pressure drop. Then, the device thickness and the saline flow rate were optimized by the response surface methodology and NSGA-II, which is one of the most popular algorithms for multi-objective optimization problems. Results: The results of steady-state simulations shown in Table 1 revealed that as the channel structure became complex, the cooling performance of the cooling device was improved and the pressure drop was increased. All cooling device models were able to lower the brain surface average temperature just below the cooling device to less than 10?. Figure 1 shows the optimization results. Device A4 achieved the highest cooling performance among the three devices and was able to lower the brain surface average temperature to 6.97?. However, compared to device C4, there was only 0.16? difference. Hence, device C4, which was able to cool the brain surface with the lowest pressure drop, was considered to be the optimum one. Conclusions: The results showed that all device models we compared had enough potential to serve as an FBC device. In addition, the optimization results showed that the branched structure was able to cool brain surface efficiently with the lowest pressure drop in the cooling device. It is necessary to conduct animal experiments to verify the validity of the simulation results. Funding: This study was supported by JSPS KAKENHI Grant Number 15H05719 and AMED Grant Number 17hm0102047h0001.
.tmb-.png?Culture=en&sfvrsn=1ab1fc20_0)