OPTIMIZING LAMOTRIGINE BLOOD LEVELS DURING CONVERSION FROM VALPROATE/LAMOTRIGINE COMBINATION TO LAMOTRIGINE MONOTHERAPY
Abstract number :
1.293
Submission category :
Year :
2002
Submission ID :
2578
Source :
www.aesnet.org
Presentation date :
12/7/2002 12:00:00 AM
Published date :
Dec 1, 2002, 06:00 AM
Authors :
Mark Sale, Barry Gidal, David Blum. Pharmacokinetic Modeling and Simulation, GlaxoSmithKline, Research Triangle Park, NC; Department of Neurology, University of Wisconsin, Madison, WI; Epilepsy Clinical Development and Medical Affairs, GlaxoSmithKline, Re
OBJECTIVE: Determine a safe method of withdrawing valproic acid (VPA) from a VPA/lamotrigine combination as evaluated by fluctuation in trough levels of lamotrigine (LTG).
VPA inhibits the clearance of LTG. The maximum inhibition of LTG clearance that can be achieved is 66%, and is achieved with VPA doses of 500mg/d. The VPA concentration resulting in half of this maximum clearance is 2.05[mu]g/ml (Gidal et al, Epilepsia 2001;42:89). Withdrawal of VPA from a LTG/VPA regimen is complicated by this interaction.
METHODS: NONMEM was used to construct a mechanistic pharmacokinetic model of the interaction of VPA and LTG (LAMICTAL[reg]). Simulations with this model were used to examine the risk of subtherapeutic or toxic concentrations for four candidate VPA withdrawal regimens. All regimens started with VPA 500 mg/d and LTG 200 mg/d, each divided twice daily. Metrics used for comparison are the median trough value for LTG and the 90% Confidence Interval for the ratio of the pretransition trough to the lowest and highest trough values achieved during the transition (ratio range). Criteria for optimal regimen selection were: 1) high minimum trough value, 2) small range, 3) ease of utilization.
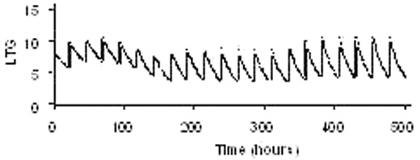
RESULTS: The trough ranges achieved with four candidate regimens are shown, and median hourly levels are graphed for the [dsquote]optimal[dsquote] regimen: [table1]Figure 1 Median LTG levels during withdrawal according to the regimen in line one of the table above[figure1]
CONCLUSIONS: Transition from a combined regimen of VPA and LTG to LTG alone can be achieved with low risk of subtherapeutic or toxic concentrations. The conversion regimen which combines smoothest control of LTG blood levels and ease of use is:
Step 1 [ndash] adjust LTG to 200 mg/d and gradually decrease VPA to 500 mg/d, maintain for 1 week.
Step 2 [ndash] Decrease VPA to 250 mg/d and increase LTG to 300 mg/d, for 1 week
Step 3 [ndash] Discontinue VPA, and make weekly increases in LTG until achieving the target dose of 500 mg/d or as clinically indicated.
[Supported by: GlaxoSmithKline Research and Development]; (Disclosure: Salary - Mark Sale and David Blum are employees of GlaxoSmithKline, Grant - Barry Gidal received grant support from GlaxoSmithKline to assist in the conduct of this study.)