Padsevonil Differs From Brivaracetam and Levetiracetam in Its Interaction With the Synaptic Vesicle 2A (SV2A) Protein
Abstract number :
2.225
Submission category :
7. Antiepileptic Drugs / 7A. Animal Studies
Year :
2018
Submission ID :
497175
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Martyn Wood, UCB Pharma and Michel Gillard, UCB Pharma
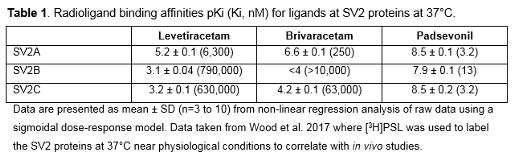
Rationale: Padsevonil (PSL; UCB0942) is a novel antiepileptic drug (AED) candidate with selective pre- and post-synaptic activity. It has high affinity for all three isoforms of the synaptic vesicle 2 (SV2) protein (Table 1) and low-to-moderate affinity for the benzodiazepine site on the GABAA receptor (Wood M, et al. AES 2017, abst 1.271). This contrasts with the properties of brivaracetam (BRV) and levetiracetam (LEV), which selectively interact with SV2A protein only. Furthermore, PSL displayed higher potency across seizure models and was active in models where LEV or BRV are not active (Leclercq K, et al. AES 2017, abst 1.272). PSL showed encouraging anticonvulsant activity in drug-resistant patients (Muglia P, et al. AES 2017, abst 1.283). We have previously shown that BRV and LEV interact differently with the SV2A protein in that the SV2A modulator UCB1244283 potentiated their binding by different mechanisms (Wood MD, Gillard M. Epilepsia 2017;58:255–62). We therefore investigated whether UCB1244283 differentially affects the interaction of PSL with the SV2A protein. Methods: [3H]PSL (2.55 Ci mmol-1) was custom-labeled (Aptuit, Kansas, USA). Recombinant human SV2A proteins were expressed in HEK 293 cells. Membranes were prepared and binding assays performed according to Gillard M et al. (Eur J Pharmacol 2011;664:36–44) and Wood MD and Gillard M (2017). Binding studies were carried out at 4°C to allow comparison to previous studies using [3H]BRV and [3H]LEV which have fast off-rates. Saturation binding assays were performed using varying concentrations of [3H]PSL (c 0.1–25 nM) and data analyzed using GraphPad PRISM with the one-site total and nonspecific binding model to determine affinity (Kd) and number of binding sites (Bmax). Results: In membranes expressing recombinant SV2A proteins, [3H]PSL displayed high affinity and saturable binding (Table 2). The presence of the modulator had no statistically significant effect on either the affinity of capacity of the binding of [3H]PSL (Table 2). Conclusions: Unlike BRV and LEV, PSL has similarly high affinities for the SV2B and SV2C proteins, in addition to its high SV2A affinity. We have shown that UCB1244283 potentiates the binding of [3H]BRV and [3H]LEV to the SV2A protein and that it does this by different mechanisms – an increase in binding capacity for LEV and an increase in affinity for BRV. We now show that the modulator surprisingly lacks any significant effect on the SV2A binding of [3H]PSL. This further suggests that PSL has a unique interaction with the SV2A protein. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=6336e4ca_0)