RELATIVE BIOAVAILABILITY AND BIOEQUIVALENCE OF THREE DIFFERENT ORAL FORMULATIONS OF ESLICARBAZEPINE ACETATE
Abstract number :
2.325
Submission category :
Year :
2005
Submission ID :
5631
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
1Teresa Nunes, 1Amilcar Falcao, 1Luis Almeida, 1Ricardo Lima, 1Susana Tavares, 2Carla Neta, 2Carlos Fontes-Ribeiro, 2Tice Macedo, and 1Patricio Soares-da-Si
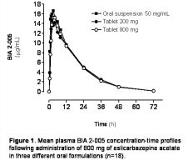
To investigate the bioavailability and bioequivalence of three formulations of eslicarbazepine acetate: 50 mg/mL oral suspension (Test 1), 200 mg tablets (Test 2) and 800 mg tablets (Reference). This was a single-centre, open-label, randomised, 3-way crossover study in 18 healthy subjects. Each subject received a 800 mg single-dose of eslicarbazepine acetate in three different occasions: either 16 mL of oral suspension 50 mg/mL, 4 tablets 200 mg or 1 tablet 800 mg. Single-doses were separated by a washout period of 7 days or more. Eslicarbazepine acetate was rapidly and extensively metabolised to BIA 2-005. Figure 1 displays mean BIA 2-005 plasma concentration-time profiles and Table I presents the main pharmacokinetic parameters maximum plasma drug concentrations (C[sub]max[/sub]), time to its occurrence (t[sub]max[/sub]), area under the plasma concentration-time curve from time zero to infinity (AUC[sub]0-[/sub][sub][infin][/sub]), and the apparent terminal half-life (t[sub][frac12][/sub]). Point estimate (PE) and 90% confidence intervals (90%CI) were calculated for the AUC[sub]0-[infin][/sub] and C[sub]max[/sub] geometric means. PE and 90%CI for AUC[sub]0-[infin][/sub] Test 1/Reference ratio were 1.09 and 1.01-1.15; for C[sub]max[/sub] ratio, PE and 90% CI were 1.07 and 0.97-1.15. When Test 2 and Reference formulations were compared, the PE and 90%CI were 0.99 and 0.94-1.07 for the AUC[sub]0-[infin][/sub] ratio, and 0.94 and 0.86-1.02 for the C[sub]max[/sub] ratio. Bioequivalence of Test versus Reference formulations is accepted for both AUC[sub]0-[infin][/sub] and C[sub]max[/sub] because the 90%CI lie within the acceptance range of 0.80-1.25. The pharmacokinetic profiles of three different formulations of eslicarbazepine acetate (oral suspension 50 mg/mL, 200 mg tablet and 800 mg tablet) were similar and these formulations can be considered bioequivalent.[figure1][table1]