Sleep Improves the Localization of the Epileptic Focus
Abstract number :
1.037
Submission category :
1. Basic Mechanisms / 1C. Electrophysiology/High frequency oscillations
Year :
2019
Submission ID :
2421033
Source :
www.aesnet.org
Presentation date :
12/7/2019 6:00:00 PM
Published date :
Nov 25, 2019, 12:14 PM
Authors :
Petr Klimes, Montreal Neurological Institute and Hospital, McGill University, Montréal, Canada; Institute of Scientific Instruments, The Czech Academy of Sciences, Brno, Czech Republic; Jan Cimbalnik, International Clinical Research Center, St. Anne’s Uni
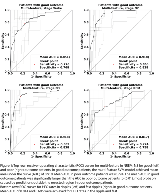
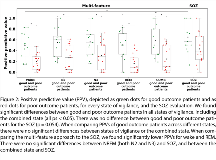
Rationale: In drug-resistant epilepsy, accurate localization of the epileptogenic zone (EZ) is crucial before its resection to achieve seizure freedom with minimal side effects. Recent studies suggested promising seizure-independent markers of the EZ, such as epileptic spikes, high-frequency oscillations, or changes in connectivity. However, these EEG features show variations across the sleep-wake cycle. This study investigates which state of vigilance is the best to localize the EZ in the interictal intracranial EEG. Methods: 30 patients undergoing stereo-encephalography (SEEG) / sleep recording and open surgery were included; 13 patients had good surgical outcome (Engel I). Sleep was scored visually following standard criteria. For the analysis, 10 minutes of wake, N2, N3, and REM were selected. Oscillatory, univariate and bivariate EEG features from good outcome patients were used to train a support vector machine (SVM) model. The oscillatory features were rates of epileptic spikes, rates of oscillatory events in frequency bands 1-4 Hz, 4-8 Hz, 8-12 Hz, and rates of high frequency oscillations (HFOs) in 65-80 Hz (high gamma band), 80-250 Hz (ripple band), and 250-600 Hz (fast ripple band). Furthermore, univariate and bivariate features were calculated in frequency bands 1-4 Hz, 4-8 Hz, 8-12 Hz, 12-20 Hz, 20-45 Hz, 65-80 Hz, 80-250 Hz, 250-600 Hz. Univariate features were spectral power and power spectral entropy. Bivariate features were linear correlation, linear correlation with lag, relative entropy, phase synchrony, phase consistency, and phase-lag index. The trained SVM model produced predictions, labeling each SEEG contact as non-EZ or EZ. Subsequently, these predictions were tested against true classifications. The performance of the algorithm was evaluated by the mean area under the receiver operating characteristic curves (AUCs) and positive predictive values (PPVs) across sections of wake, N2, N3, REM, and their combination. Results: In good outcome patients, the multi-feature SVM model achieved mean AUCs of 0.85 in wake, 0.92 in N2, 0.92 in N3, 0.82 in REM, and 0.90 in the combined state. The AUCs achieved in NREM sleep stages N2 and N3 were higher when compared to wakefulness and REM sleep (p<0.01). The highest average PPV = 0.56 was found for NREM sleep stage N3. There was no improvement when using a combination of all states (PPV = 0.58; p>0.05). The majority of selected features were based on either relative entropy or HFOs. In the combined state, the SVM algorithm selected eight out of ten best performing features from NREM sleep. The multi-feature approach outperformed HFO rates as single marker (p<0.01), and its result was not different from the seizure-onset zone (SOZ, p>0.05), which was based on 12.7 days of seizure monitoring (p>0.05). Conclusions: Sleep improves the localization of the EZ with best identification achieved in NREM sleep stages N2 and N3. Results obtained from 10 minutes showed similar results as a combination of the different states of vigilance or the SOZ as traditional proxy for the EZ, which requires multiple days of recording and hours of visual inspection. Our results also support previous findings, showing that SVM models combining multiple EEG features show better performance than algorithms using single EEG markers. This finding might ultimately result in a more time-efficient intracranial presurgical investigation of focal drug-resistant epilepsy. Funding: Molson Neuroengineering fellowship of the MNI, Inter-Action (LTAUSA18), Fonds de Recherche du Québec – Santé, Canadian Institutes of Health Research (FDN 143208).