STEADY-STATE BIOEQUIVALENCE OF 25-, 50-, AND 100-mg ZONISAMIDE CAPSULES GIVEN AT EQUAL DOSES IN HEALTHY ADULT VOLUNTEERS
Abstract number :
1.312
Submission category :
Year :
2004
Submission ID :
4340
Source :
www.aesnet.org
Presentation date :
12/2/2004 12:00:00 AM
Published date :
Dec 1, 2004, 06:00 AM
Authors :
1James C. Cloyd, 2Martin J. Brodie, and 3John Grundy
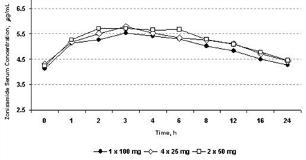
Zonisamide was approved in the United States with a single dose strength (100-mg capsules). Subsequent clinical experience has shown that initiation of zonisamide at dosages less than 100 mg/d and maintenance dose adjustments less than 100 mg/dose are useful in optimizing therapy. In order to facilitate better therapeutic titration and improve treatment flexibility, new zonisamide dose strengths (25- and 50-mg capsules) have been developed. This study compared the bioavailability of these new formulations to that of the 100-mg capsules using equipotent dosages. This single-center, open-label, 3-period, randomized, crossover, steady-state study was conducted to establish bioequivalence of four 25-mg or two 50-mg capsules with a single 100-mg capsule of zonisamide, each given once daily, in healthy adults. Eighteen subjects completed three 14-day periods of zonisamide treatment, 1 period for each of the 3 treatments. Attainment of steady state was assessed by comparing zonisamide predose trough concentrations on Days 12 through 14 to those on Day 15 for each treatment. Serum zonisamide concentrations were also measured over a 24-hour interval on Day 14 of each period using a high performance liquid chromatography method to determine minimum steady-state plasma concentration (C[sub]min,ss[/sub]), maximum steady-state plasma concentration (C[sub]max,ss[/sub]), time to maximum steady-state plasma concentration (t[sub]max,ss[/sub]), and area under the time-concentration curve (AUC[sub]t[/sub]). Steady state was achieved by Day 13 for each capsule strength. The pharmacokinetic parameters C[sub]min[/sub], C[sub]max[/sub], t[sub]max[/sub], and AUC[sub]t[/sub] were similar for the 3 dosage regimens. Serum zonisamide concentrations for the 3 dosing regimens on Day 14 are shown in Figure 1. [figure1] Bioequivalence was established for both the 25 mg x 4 and 50 mg x 2 treatments, compared with a 100-mg capsule because the geometric mean ratio estimates and associated 90% confidence intervals of log-transformed C[sub]max,ss[/sub] and AUC[sub]t [/sub]values were within the interval of 80% to 125%. Four 25- or two 50-mg zonisamide capsules once a day were found to be bioequivalent to a single 100-mg capsule, demonstrating dose-form proportionality. The lower-strength capsules will facilitate initiation of zonisamide therapy and permit small adjustments in maintenance dosages. (Supported by Eisai Inc.)