THE IMPACT OF PREGNANCY AND CHILDBIRTH ON THE ELIMINATION OF LEVETIRACETAM
Abstract number :
1.216
Submission category :
Year :
2005
Submission ID :
5301
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
1Page B. Pennell, 1Archana Koganti, 1Sandra Helmers, 1Aquila Beach, 1Melanee Newman, 2Donald J. Newport, and 2Zachary N. Stowe
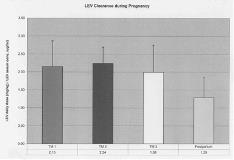
Ideal treatment of women with epilepsy during pregnancy involves achieving an optimal balance between maintaining seizure control and minimizing fetal exposure to AEDs. Studies of older AEDs and LTG have demonstrated lower serum concentrations during pregnancy, often with worsened seizures. Other second generation AEDs have not been studied as extensively, and data on levetiracetam (LEV) is lacking. The distinctive properties of LEV ([lt]10% protein binding and primary routes of elimination via renal excretion and extrahepatic hydrolysis) make it less susceptible to the enhanced CYP450/UGT metabolism described during pregnancy. However, renal blood flow does increase early in pregnancy and persists to term. Five pregnant women treated with LEV for epilepsy were followed in a prospective, observational study. After obtaining informed consent, patient weights, dosing regimens, and serum samples were obtained qmonth-qtrimester during pregnancy and up to 6 mos postpartum (PP) (n= 38 samples). To adjust for weight and dosage changes during pregnancy, we calculated Clearance as LEV daily dose (mg)/ body weight (kg) / serum LEV concentration (ug/mL). Mean clearance and standard deviations were calculated for each trimester and the PP stage. PP samples were considered to reflect baseline clearance rates. Delivery and newborn information were also obtained. LEV serum concentrations were obtained in 3 non-nursing infants to evaluate neonatal clearance. LEV clearance ((mg/kg)/(ug/ml)) during each perinatal interval are reported as means and standard deviations and are depicted in the figure: 1st TM 2.15 [underline]+ [/underline]0.72, 2nd TM 2.24 [underline]+[/underline] 0.45, 3rd TM 1.99 [underline]+[/underline] 0.75, and postpartum 1.29 [underline]+[/underline] 0.57. Obstetrical and fetal complications were limited: 1 mother developed preeclampsia and her infant was mildly lethargic for 2 days, and 1 newborn was small for gestational age and required supplemental oxygen. No major malformations were detected. All 3 newborn samples (1.7-3 weeks of life) did not have detectable levels of LEV. These preliminary results suggest that LEV elimination is enhanced during pregnancy in concordance with increased renal blood flow. Neonatal outcomes were favorable, and neonatal clearance was complete within the first few weeks of life. Therapeutic drug monitoring (TDM) of LEV may be warranted during pregnancy and parturition. Formal pharmacokinetic modeling of LEV during pregnancy is ongoing.[figure1] (Supported by a Specialized Center of Research P50 MH 68036.)