Tolerability of Perampanel in Pediatric Patients: A Review of Our Experience
Abstract number :
1.318
Submission category :
7. Antiepileptic Drugs / 7D. Drug Side Effects
Year :
2018
Submission ID :
504087
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Carlos Lastra, The Children's Hospital at Saint Peter's University Hospital; Evan Khan, The Children's Hospital at Saint Peter's University Hospital; and Chandrabhaga Miskin, The Children's Hospital at Saint Peter's University Hospital
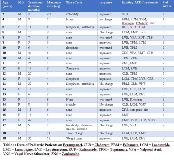
Rationale: Perampanel (Fycompa) is a newer antiepileptic drug (AED) approved by the FDA as monotherapy or adjunctive therapy for partial-onset seizures with or without secondary generalization and primary generalized tonic-clonic seizures in patients older than 12 years of age. Perampanel (PER) is a novel AED due to its mechanism of action as a non-competitive, selective AMPA receptor antagonist (1) and a favorable cognitive profile. There is limited data on the use of PER in pediatric patients younger than 12 years of age (2). Our study is a review of tolerability (side effects) in all pediatric patients treated with PER in our institution Methods: Charts of patients being prescribed PER were obtained by using the electronic medical record system. Patients 18 years of age and younger were selected. Data was collected according to age, gender, highest PER dose, duration of treatment, and additional AEDs or other treatments for epilepsy. The charts were reviewed for reported side effects and PER discontinuation. Efficacy was measured by chart notation of seizure frequency, although percentage of seizure improvement could not be used due to no uniform seizure record keeping. Results: A total of 24 patients were prescribed PER. The mean age was 11.8 SD +/- 4.02 (median 12.5 range of 2 - 18 years), including 14 females and 10 males. The mean duration of treatment was 14.6 months with a range of 2 months to 42 months. Average number of concomitant AEDs was 2.6 with a range of 1-4 AEDs. Three patients had a vagal nerve stimulator and one patient was on a ketogenic diet. All patients have refractory epilepsy, including Dravet and Lennox-Gastaut Syndrome. 10 patients were younger than 12 years (51%). Reported side effects were found in 9/24 patients (37.5%) and the most frequent side effects corresponded to irritability 5/24 (20.8%) and sleepiness 5/24 (20.8%), dizziness 1/24 (4.2%). These side effects have been reported previously as the most common. 1/24 (4.2 %) reported suicidal ideation causing PER discontinuation. Other side effects reported; elevated blood pressure in one patient, temperature instability in another patient, and weight gain in another, explained by other causes than PER. 15/24 (62.5%) reported no side effects. Side effects found in only 2/10 (20 %) of the patients younger than 12 years of age; one patient complaining of irritability and sleepiness and the other one sleepiness. Conclusions: These findings are consistent with other reports of PER side effects. Irritability and sedation were the most frequent side effects in this review. Dizziness was not a frequent side effect; although is reported in 20 % of patients 12 years and older (2). Overall, PER was well tolerated and improved seizure frequency in more than half of the patients. Other variables were not analyzed in this study including dose, duration of treatment, number of AEDs, interaction with other medications. Therefore, prospective studies with a larger number of younger patients are needed to assess side effects and seizure control. References1. Rogawski MA, Hanada T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol Scand Suppl. 2013;197:19–24.2. Swiderska N, Tan HJ, Rajai A, Silwal A, Desurkar A, Martland T. Effectiveness and tolerability of Perampanel in children, adolescents and young adults with refractory epilepsy: A UK national multicentre study. Seizure. 2017 Nov;52:63-70. doi: 10.1016/j.seizure.2017.08.014. Epub 2017 Sep 14 Funding: No funding
.tmb-.jpg?Culture=en&sfvrsn=d3d17892_0)