Yield of Whole Exome Sequencing: Experience from an Epilepsy Genetics Program
Abstract number :
2.372
Submission category :
12. Genetics / 12A. Human Studies
Year :
2018
Submission ID :
501013
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Egidio Spinelli, Lurie Children's Hospital of Chicago; Emily M. Bryant, Lurie Children's Hospital of Chicago; Jessica Nowicki, Lurie Children's Hospital of Chicago; Rebecca Garcia Sosa, Lurie Children's Hospital of Chicago; Sunita N. Misra, Ann & Robert H
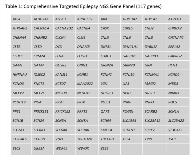
Rationale: A large proportion of patients with presumed genetic causes of epilepsy have negative testing using traditional investigative methods such as metabolic testing, chromosome microarray or next generation sequencing (NGS) panels of selected genes. For these patients, Whole Exome Sequencing (WES) has become a powerful tool to facilitate diagnosis, potentially aide with management of seizures and guide future family planning. Methods: All patients seen at the Epilepsy Genetics Clinic at our hospital from April 2017 to April 2018 and who received WES testing were enrolled. Patients were referred to the clinic when initial evaluation with history, exam, neuroimaging, EEG and other testing was inconclusive. The clinic has a standardized testing algorithm whereby patients are initially evaluated with an in-house targeted 117 gene (Table 1) NGS epilepsy panel. This panel does not include gene deletion-duplication analysis. If testing was unrevealing and further testing was indicated, WES by a commercial laboratory was then recommended. Charts were reviewed for information regarding epilepsy diagnosis and WES results. Results: WES results were available in 50 patients (28 girls, 22 boys, median age at testing 7 y, range 3 m to 17 y). Seizure onset ranged from < 1 m to 12 y. Epilepsy diagnoses were characterized as epileptic encephalopathy, genetic generalized epilepsy, focal epilepsy of unknown etiology and epilepsy secondary to a structural lesion. Thirteen (26%) patients had either pathogenic or likely pathogenic variants. Fifteen (30%) patients had variants of unknown significance. In twenty-two (44%) patients, WES failed to identify the pathogenic variant in these individuals. Pathogenic variants with potential therapeutic implications included: SLC2A1 (Glucose Transporter Deficiency), PHGDH (Serine Deficiency), KCNT1 (Epilepsy of Infancy with Migrating Focal Seizures), ATP1A2 (Alternating Hemiplegia of Childhood) and AMT (non-ketotic hyperglycinemia). Three patients required specialist referral and/or counselling for non-epilepsy related genetic findings. Conclusions: WES is a powerful tool in the diagnosis and management of epilepsy. A comprehensive epilepsy genetics clinic with genetic counseling, focused testing and clinical management by experts can provide families with comprehensive care for these rare conditions. Funding: None