Compliance With the Quality Measure for Epileptic Spasms
Abstract number :
3.207
Submission category :
4. Clinical Epilepsy / 4C. Clinical Treatments
Year :
2018
Submission ID :
505719
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
John R. Mytinger, Nationwide Children's Hospital
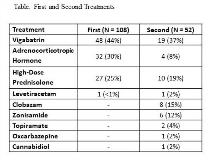
Rationale: The 2017 AAN/CNS child neurology quality measure set includes the initial treatment of epileptic spasms (ES) with adrenocorticotropic hormone (ACTH), high-dose prednisolone (HP) or vigabatrin (VGB) unless refused, contraindicated, enrolled in research or better treated with surgery. As part of an ongoing quality improvement (QI) initiative started in 2012, the aim of the current analysis is to determine the percentage of patients receiving ACTH, HP or VGB for both initial and second treatments for ES and to alter practice if indicated. While second treatment is not part of the quality measure, we included this given that nearly half of patients treated with ACTH, HP or VGB as the second treatment achieve remission. Methods: There were 127 patients treated for ES at our center from 9/2012 to 5/2018. Nineteen who received initial treatment at an outside hospital were excluded leaving 108 for analysis. Otherwise, all patients with confirmed ES ages 2 to 36 months were included. Data was gathered by retrospective medical record review. At our center, combination therapy is not used. Formal IRB review confirms exemption for QI. Results: ACTH, HP or VGB was used as the initial treatment in 107/108 (99%). One received levetiracetam for parent refusal of ACTH, HP and VGB. The most commonly used initial treatment was VGB. Fifty-two were eligible for a second treatment and the treatment was ACTH, HP or VGB in 33/52 (63%). The most commonly used second treatment was VGB. Treatments are presented in the Table. Of the 19 patients who did not receive ACTH, HP or VGB as the second treatment, 7 were not eligible for hormone (all initially received VGB), 5 refused and 1 was enrolled in a research trial. Thus, 6 were eligible for ACTH, HP or VGB but did not receive it as the second treatment. Conclusions: We had complete compliance with the quality measure of using ACTH, HP or VGB as the initial treatment for ES. This suggests that compliance can be achieved using a standardized guideline even within a large tertiary care center where the care of ES patients is shared among all faculty and residents. In contrast to a 2012 survey reporting ACTH as the most commonly preferred initial treatment for ES, VGB was the most commonly used initial and second treatment at our center. The frequent use of VGB may relate to the combination of easy acquisition (15 day starter pack) and administration (compared to ACTH) as well as shorter hospital stay (compared to ACTH) and relative safety (compared to ACTH and HP). Although ES remission may occur with ACTH after failing HP, of the 16 patients who received HP, only 2 patients received ACTH as the second treatment. Only 2 patients received ACTH after VGB. Clobazam (8) was a more common second treatment than ACTH (4). We identified 6 patients who were eligible for ACTH, HP or VGB as the second treatment but did not receive it. Changes in practice may include greater utilization of ACTH after failed HP or VGB and to assure patients receive ACTH, HP or VGB as the second treatment if possible. This is the first report of compliance with the new ES quality measure. Funding: This is an unfunded project.