Computationally Targeted Non-Invasive Neurostimulation Provides Meaningful Reduction in Seizure Frequency in Adult and Pediatric Patients Not Responsive to Medication
Abstract number :
1.225
Submission category :
4. Clinical Epilepsy / 4C. Clinical Treatments
Year :
2018
Submission ID :
499079
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Giulio Ruffini, Neuroelectrics Inc.; Harper L. Kaye, Boston Children's Hospital, Harvard Medical School; Carolina Santiago, Neuroelectrics Inc.; Ricardo Salvador, Neuroelectrics Inc.; Paul Pyzowski, Neuroelectrics Inc.; León Morales Quezada, Spauldin
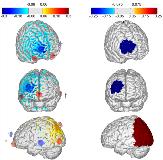
Rationale: Implanted neurostimulation devices including deep-brain stimulation (DBS) and responsive neuromodulation (RNS) have been demonstrated to be effective in reducing seizures in patients not responsive to medication. However these devices and related surgical procedures are expensive, invasive, and have a substantial non-responder rate. Non-invasive neuromodulation using externally applied electric currents could play an important role in the treatment of epilepsy. Transcranial current stimulation (tCS) is a non-invasive technique that delivers low levels of electric currents to the brain via scalp electrodes. Unlike implanted neurostimulation systems, tCS delivers subthreshold stimulation; with repeated use (typically 20 minutes per day, over several days) the neurons see a persistent increase in activation threshold, and a resulting decrease in firing activity. Consequently, tCS may be useful in decreasing seizures. However, limitations in the ability to deliver stimulation preferentially to the the seizure focus/foci have limited its clinical use to date. Methods: We have developed methods and software to allow precise personalized targeting of transcranial current stimulation. First, a computational model of the anatomy of the brain is developed using mathematical finite-element models, and can be based on the individual MRI of the patient. Location of the seizure foci is specified by the treating neurologist. Next, a computer algorithm is used to determine the placement of up to eight independent electrodes on the scalp, to optimize the levels and orientation of currents.A treatment protocol was developed where patients are treated with ten 20-minute stimulation sessions over two weeks. Seizure frequency is measured for eight weeks prior (to establish baseline) and after the stimulation sessions. Patients remain on previously prescribed medications during the protocol.This method was tested in an open-label study under an FDA approved investigational device exemption (IDE) at Boston Children’s Hospital, with adult patients referred by Beth-Israel Deaconess Medical Center (Boston). Additional patients were treated following the same protocol at the National Institute of Neurology and Neurosurgery (Mexico City). Results: Fourteen patients with medically-refractory focal epilepsy of varying etiologies were enrolled in the protocol; two dropped out before the end of the two-week treatment window due to self-reported increases in seizure frequency, with return to baseline soon (within 2 weeks) after the end of stimulation. Of the twelve patients that completed the protocol, the mean decrease in seizure frequency was 56% during the treatment, 48% four weeks post-treatment, and 42% eight weeks post-treatment. There were no serious adverse events reported during the study, and several patients noted marked improvements in quality of life measures. Conclusions: Targeted personalized non-invasive neurostimulation may be be effective in reducing seizures in patients with refractory focal epilepsy. The authors intend to continue this work in a blinded, placebo-controlled study under an FDA IDE for eventual device approval. Funding: Funding was provided by Neuroelectrics Inc. and Massachusetts Life Sciences Center.
.tmb-.jpg?Culture=en&sfvrsn=34cdc255_0)