Long-Term Safety and Efficacy of Lacosamide and Controlled-Release Carbamazepine Monotherapy in Patients with Newly Diagnosed Epilepsy
Abstract number :
1.291
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
497185
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Elinor Ben-Menachem, Institute for Clinical Neuroscience & Physiology, University of Gothenburg; Hans Peter Grebe, Centro Hospitalar de Entre Douro e Vouga, Santa Maria da Feira, Portugal; Kiyohito Terada, Shizuoka Institute of Epilepsy and Neurological D
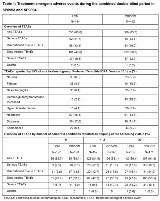
Rationale: In a large-scale, double-blind trial (SP0993; NCT01243177), lacosamide (LCM) was non-inferior to controlled-release carbamazepine (CBZ-CR) and was well tolerated as first-line monotherapy by patients aged ≥ 16 years with newly diagnosed epilepsy (Baulac et al. Lancet Neurol 2017;16:43–54). This post-hoc analysis explored long-term, clinically relevant outcomes during LCM and CBZ-CR monotherapy in adults with newly diagnosed epilepsy. Methods: Data were pooled from SP0993 and the double-blind extension (SP0994; NCT01465997). SP0993 enrolled adults (≥ 16 years) with newly diagnosed epilepsy (focal or generalized tonic-clonic seizures [without clear focal or generalized origin]). LCM and CBZ-CR were initiated at 100mg/day or 200mg/day, respectively, and titrated to a target dose of 200 mg/day (LCM) or 400 mg/day (CBZ-CR), with increases to 400/600 mg/day (LCM) and 800/1200 mg/day (CBZ-CR) if needed. Patients achieving 6-month seizure freedom at the last evaluated dose level were eligible for SP0994 after achieving 12-month seizure freedom or discontinuing after experiencing a seizure. The safety and efficacy outcomes during the combined double-blind period in SP0993 and SP0994 were explored, including an analysis by number of comorbid conditions. Results: 444 and 442 patients received LCM and CBZ-CR, respectively. Median treatment duration was slightly longer in the LCM (630 days) vs CBZ-CR (589 days) group. The Kaplan-Meier estimated proportion of patients who were seizure free for 12 and 24 months from the first dose were 50.8% (95% CI: 46.2 – 55.4%) and 47.0% (95% CI: 42.2 – 51.7%) on LCM and 54.9% (95% CI: 50.3 – 59.6%) and 50.9% (95% CI: 46.0 – 55.7%) on CBZ-CR, respectively. For patients with ≥ 24 months of treatment (LCM n=177; CBZ-CR n=164), 98.9% on LCM and 99.4% on CBZ-CR had at least 12 months seizure freedom; 84.2% on LCM and 82.9% on CBZ-CR had 24 months seizure freedom. Fewer patients on LCM vs CBZ-CR experienced drug-related TEAEs or discontinued due to TEAEs overall (Table 1). The incidence of drug-related TEAEs and discontinuations due to TEAEs increased by number of comorbid conditions and was consistently lower in patients on LCM (Table 1). These findings were supported by the Kaplan-Meier estimated proportion of patients who did not discontinue treatment due to TEAEs at 1- and 2-years (Table 2). Conclusions: Long-term LCM monotherapy (median duration of ~2 years) was efficacious and generally well tolerated in adults with newly diagnosed epilepsy. Seizure freedom rates were similar in patients on LCM and CBZ-CR. Fewer patients on LCM experienced drug-related TEAEs or discontinued due to TEAEs vs patients on CBZ-CR. Drug-related TEAEs and discontinuations due to TEAEs increased by number of comorbid conditions and were consistently lower in patients on LCM. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=21b644c5_0)