Response to Lacosamide Monotherapy in a Patient With Medically Refractory Jeavons Syndrome
Abstract number :
2.439
Submission category :
18. Case Studies
Year :
2018
Submission ID :
501898
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Ifrah Zawar, Cleveland Clinic Foundation; Linda Franic, Cleveland Clinic Foundation; and Elia Pestana Knight
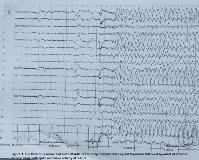
Rationale: Jeavons syndrome or eyelid myoclonia with absences (EMA) is a childhood generalized epileptic condition clinically characterized by eyelid myoclonia with or without absences, eye-closure induced electroencephalogram (EEG) paroxysm, and photosensitivity. EEG shows brief generalized polyspikes or generalized spike wave activity at 3-6 Hz triggered by eyelid closure which may disappear in darkness. It is generally treated with valproic acid, ethosuximide, benzodiazepine, lamotrigine and levetiracetam either as monotherapy or in combination. To our knowledge, there are no previous reports of lacosamide use in medically refractory Jeavons syndrome. In this case, we describe a case of medically refractory Jeavons syndrome with highly favorable response to lacosamide monotherapy. Methods: Case Report & Literature Review Results: A 14-year-old female with Attention Deficit Hyperactivity Disorder and Oppositional Defiant Disorder presented with uncontrolled seizures that initially started at 9 months of age. On the basis of her initial EEG findings and seizure semiology, she carried the diagnosis of Childhood Absence Epilepsy since the age of 3. Between the ages of 3 and 12 years, she was given adequate trials of various medications, including ethosuximide, valproic acid, lamotrigine, topiramate, and ketogenic diet both as monotherapy and in combination but her seizures could not be controlled. Between the ages of 12 to 14 years, she remained off anti-epileptic drugs because of poor seizure control on medications and medication side effects. She continued to have multiple daily seizures. At the age of 14, she was admitted to Epilepsy Monitoring Unit to clarify the diagnosis of her epileptic syndrome as Childhood Absence Epilepsy mostly resolves by this age. During a 24 hour of video EEG monitoring, we captured 4 clusters of her typical seizures. On EEG, normal awake and sleep background rhythms were interrupted by bursts of generalized polyspikes and generalized spike wave complexes of 3-4 Hz, most of which were elicited by eyelid closure (EEG paroxysms), with most of these bursts associated with eyelid myoclonia. She was noted to have multiple eyelid myoclonia some of which evolved into absence seizures. Some of these seizures were activated by hyperventilation. These EEG changes associated with eyelid closure were not evident in the dark. Hence her epilepsy was re-defined as Jeavons syndrome.Since she had failed multiple medications in the past, it was decided to give her a trial of lacosamide. She was loaded with 3.5 mg/kg (200 mg) of lacosamide and started at a maintenance dose of 1.75mg/kg/dose (100 mg) twice daily. She tolerated the initial doses of lacosamide well with no significant side effects and EEG showed a dramatic response with resolution of seizures and dramatic improvement in interictal discharges. Conclusions: There is limited research on anti-epileptic drug treatment for Jeavons syndrome. Valproic acid, ethosuximide, benzodiazepine, lamotrigine and levetiracetam have been used with variable efficacy. We present a case of medically refractory Jeavons syndrome (EMA) with seizure resolution in response to lacosamide monotherapy at standard daily doses. This highlights the potential role of lacosamide as an option in this syndrome if other drugs are ineffective or not tolerated. Moreover, given favorable side effect profile and low teratogenic potential, we propose that lacosamide could potentially be used as one of the initial treatment options for Jeavons syndrome in certain patient populations or for those whose comorbid conditions do not allow the use of other drugs. However, prospective controlled studies are needed to better elucidate efficacy and potential utility of this drug in this syndrome. Funding: None
.tmb-.jpg?Culture=en&sfvrsn=8d3303c9_0)