Resting-State Functional Connectivity Using Stereo-EEG Helps Identify Epileptogenic Brain Regions
Abstract number :
2.091
Submission category :
3. Neurophysiology / 3G. Computational Analysis & Modeling of EEG
Year :
2018
Submission ID :
501468
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Sarah E. Goodale, Vanderbilt University Medical Center; Hernan F.J. Gonzalez, Vanderbilt University; Kanupriya Gupta, Vanderbilt University Medical Center; Hong Yu, Vanderbilt University Medical Center; John D. Rolston, University of Utah; Victoria L. Mor
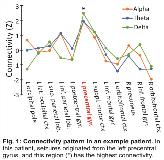
Rationale: Although stereotactic electroencephalography (SEEG) is minimally invasive and safe for seizure localization, it nonetheless requires several days to weeks in the hospital with interventions taken to trigger and record several uncomfortable seizures. Also, some patients do not have seizures during an extended admission, or clinical interpretation may be unclear. Identifying epileptogenicity using brief, resting-state recordings would be clinically useful and may shorten hospital admissions. While our previous work using magnetoencephalography has suggested increased alpha-band imaginary coherence at epileptogenic brain regions (Englot et al., 2015, Brain 138(Pt 8):2249-62.), it is unknown if this measure would be valuable using SEEG. Methods: We evaluated 15 focal epilepsy patients (35.2 ± 11.4 years old, mean ± SD; 11 females) with intracranial SEEG (sampled at 1000 Hz) for 9.8 ± 4.3 days per admission. Epileptogenic and non-epileptogenic regions (13.5 ± 3.1 structures sampled per patient) were identified using traditional clinical interpretation. In addition, 20 min of recordings during the resting state with eyes closed were collected. A 2-min segment free of epileptic spikes or artifact was selected, and imaginary coherence in the alpha, theta, and delta bands were measured between each area and other brain regions to estimate the functional connectivity of each structure. Results: Mesial temporal lobe epilepsy was identified in 11 patients (6 bilateral epileptogenicity) while 4 individuals had focal neocortical epilepsy. Large increases in alpha-band imaginary coherence were found in epileptogenic regions compared to non-epileptogenic areas (p < 0.01), and increases in theta-band (p = 0.05) and delta-band (p < 0.05) imaginary coherence were also noted in these structures (paired t-tests with Bonferroni-Holm correction, N = 15 patients). The connectivity pattern for an example patient is shown in Fig. 1, and summary data are in Fig. 2. Overall, 13 of 15 (87%) patients demonstrated higher alpha connectivity in epileptogenic areas. Across individual brain regions, increased alpha connectivity predicted epileptogenicity with a sensitivity of 80.0% (63.1% specificity), and the lack of increased alpha connectivity ruled out epileptogenicity with a negative predictive value of 92.7%. Furthermore, epileptogenic regions had larger variance between alpha, theta, and delta connectivity values (31.4 ± 36.1%) than non-epileptogenic areas (1.8 ± 1.8%; p < 0.01, paired t-test). Across non-epileptogenic regions, connectivity measures did not differ between mesial temporal and neocortical areas. Conclusions: Resting-state functional connectivity measurements may help predict epileptogenicity of brain structures using brief SEEG recordings without epileptic activity. In particular, low alpha imaginary coherence strongly suggests that a region is not epileptogenic. Connectivity measurements may improve our ability to identify epileptogenic regions ahead of definitive epilepsy surgery, and may lead to an improved understanding of brain network perturbations in epilepsy. Funding: NIH R00 NS097618 (DJE) and R01 NS075270 (VLM)
.tmb-.jpg?Culture=en&sfvrsn=18747231_0)