What Defines “Clinical Meaningful Changes in Seizure Frequency?” Analysis of Data From a Phase 3 Clinical Trial of ZX008 in Dravet Syndrome
Abstract number :
3.202
Submission category :
4. Clinical Epilepsy / 4C. Clinical Treatments
Year :
2018
Submission ID :
504807
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Rima Nabbout, Hôpital Universitaire Necker - Enfants Malades, Service de Neurologie Pédiatrique Centre de Référence Épilepsies Rares (CReER); Joseph Sullivan, University of California, San Francisco Benioff Children’s Hospit
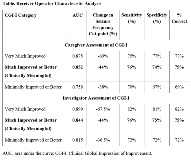
Rationale: A 50% reduction in seizure frequency is most often cited as clinically meaningful but is largely empirically derived. We set out to scientifically determine a clinically meaningful change in seizure frequency using data from a Phase 3, randomized, double-blind, placebo-controlled clinical trial of ZX008 (fenfluramine HCl oral solution) for the adjunctive treatment of seizures associated with Dravet syndrome (DS; Study 1); we utilized an anchor-based approach, examining percentage change in seizure frequency, and caregiver and investigator ratings of Clinical Global Impression of Improvement (CGI-I). Methods: Patients with DS (N=119) were enrolled and randomized to placebo, ZX008 0.2 mg/kg/day, or ZX008 0.8 mg/kg/day (1:1:1), and entered a 2-week titration followed by a 12-week maintenance period (T+M). Study 1 met its primary endpoint, with patients in the ZX008 0.8-mg/kg/day group demonstrating a 63.9% greater reduction in seizure frequency vs the placebo group. After the 14-week T+M period, caregivers and investigators rated the change in clinical status from baseline using the CGI-I scale, a validated, 7-point scale with responses ranging from 1 (very much improved) to 7 (very much worse). Patients with CGI-I scores of 1 (very much improved) or 2 (much improved) were considered to have achieved a clinically meaningful response; a score of 3 (minimally improved) was not considered meaningful as most clinicians and caregivers desire a better response. The results of the three treatment groups were pooled for this analysis. The clinically meaningful percentage change in seizure frequency was estimated by receiver operating characteristic (ROC) analysis of binary CGI-I score vs percentage change in seizure frequency, and was defined as the cut-point for which specificity and sensitivity were equal or most similar. Results: CGI-I assessments were provided for 112 patients by caregivers and 114 patients by investigators. ROC analysis identified a 44% reduction in seizure frequency as the clinically meaningful cutoff point (Table) for both caregiver and investigator assessments. Based on this threshold, 75%, 46%, and 12.5% of patients in the ZX008 0.8-mg/kg/day, ZX008 0.2-mg/kg/day, and placebo groups, respectively, achieved a clinically meaningful reduction from baseline in seizure frequency in Study 1. Conclusions: This analysis of the association between percentage change in seizure frequency and CGI-I rated by caregivers or investigators suggests that a 44% reduction from baseline in seizure frequency can be considered a clinically meaningful response in patients with DS. Notably, higher levels of seizure reduction (60%-68%) were associated with caregiver and investigator CGI-I ratings of “very much improved.” Further analyses from other Phase 3 studies in DS and other patient populations should be performed to confirm these findings and explore other potential factors that contribute to caregiver and investigator CGI-I ratings, such as non-seizure outcomes and tolerability. Funding: Zogenix, Inc.