A DOUBLE-BLIND, ADD-ON, PLACEBO-CONTROLLED EXPLORATORY TRIAL OF ESLICARBAZEPINE ACETATE IN PATIENTS WITH PARTIAL-ONSET SEIZURES
Abstract number :
2.229
Submission category :
Year :
2005
Submission ID :
5535
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
Luis Almeida, Joana Maia, and Patricio Soares-da-Silva
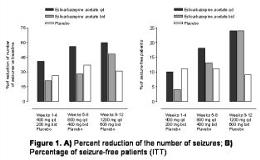
To explore the efficacy and safety of eslicarbazepine acetate (BIA 2-093), a new voltage-gated sodium channel blocker, as adjunctive therapy in adult patients with partial epilepsy. Multicenter, double-blind, randomized, placebo-controlled, therapeutic exploratory study in 143 patients aged 18-65 years with at least 4 partial-onset seizures per month in spite of treatment with 1 or 2 standard antiepileptic drugs. The study consisted of a 12-week treatment period followed by a 1-week tapering off. Patients were randomly assigned to one of three groups: treatment with eslicarbazepine acetate once-daily (OD, n=50), twice-daily (TD, n=46) or placebo (PL, n=47). For weeks 1-4, daily dose was 400 mg; then, doses were up-titrated to 800 mg and 1200 mg at 4-week intervals. Statistical analysis was performed in the intent-to-treat (ITT) population (all patients with at least one administration of study medication). Proportion of responders (patients with a [ge]50% reduction in seizure frequency) had been defined as primary endpoint. The percentage of responders at the end of the treatment period versus baseline showed a statistically significant difference between OD and PL (54% versus 28%); the difference between the TD (41%) and PL did not reach statistical significance. During weeks 1-4 (400 mg), no significant differences in the responder rate were found. During weeks 5-8 (800 mg), proportion of responders reached 58% in the OD and 38% in the PL groups; no statistical difference between TD and PL was found. A significantly higher proportion of responders in weeks 5-8 was found in OD versus TD (58.0% versus 32.6%, p=0.022). Reduction in number of seizures during each 4-week period compared to baseline is presented in Figure 1A. At each 4-week period, OD showed a significantly higher reduction in the seizure number than PL (p=0.037, p=0.018 and p=0.002, respectively). During the 12-week treatment phase, the number of seizure-free patients increased in both eslicarbazepine acetate treatment groups (OD and TD) (Figure 1B). The incidence of adverse events was similar between the treatment groups. The most reported adverse events were nausea, headache, dizziness and somnolence. Most adverse events were mild in intensity and no drug-related serious adverse events occurred. Eslicarbazepine acetate was found to be effective and well tolerated as adjunctive therapy of partial-onset seizures refractory to treatment with 1 or 2 anti-epileptic drugs. Eslicarbazepine acetate tended to be more effective when administered once-daily than when administered divided in two doses.[figure1]