A Novel Pathogenicity Modelling Approach Allows Differentiation Between Mild and Severe Missense Variants in SCN1A-Related Epilepsies
Abstract number :
1.397
Submission category :
12. Genetics / 12A. Human Studies
Year :
2018
Submission ID :
501735
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Ismael I. Ghanty, University of Glasgow and Royal Hospital for Children; Eduardo Pérez-Palma, University of Cologne; Stephanie Schorge, UCL School of Pharmacy; Joseph D. Symonds, Royal Hospital for Children, Glasgow; Sameer M. Zuberi, University of G
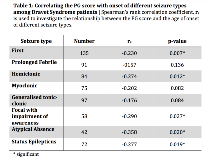
Rationale: Voltage-gated sodium channel genes are implicated in several childhood-onset epilepsies and their channels are very similar in structure. Variants in the SCN1A gene are associated with a spectrum of epilepsy phenotypes ranging from mild genetic epilepsy with febrile seizures plus (GEFS+) to the severe Dravet syndrome (DS). Currently, when a SCN1A missense variant is detected in a young child it is not possible to predict the disease trajectory. Here, we present a novel approach for classifying missense variants. Methods: “Paralog conservation”, a measurement of the conservation of an amino acid (AA) residue at a specific position across the 10 human sodium channel a-subunit genes, is combined with the physicochemical property change of the AA substitution to generate a Paralog*Grantham (PG) score. This is used to characterise SCN1A missense variants in relation to the epilepsy phenotype.To validate our approach, we collected all published electrophysiological studies of SCN1A missense variants and correlated these with corresponding PG scores. Results: The median PG score was significantly higher for DS missense variants (n = 139) compared to GEFS+ missense variants (56 vs 33, n = 24; p = 0.004). Within the group of DS patients with missense variants, a high PG score was associated with a significantly earlier onset of seizures including the age at first seizure (p=0.007), hemiclonic seizures (p=0.012), status epilepticus (p=0.019) and atypical absences (p=0.020; table 1).Based on the median PG score we divided missense variants into 2 groups, a mild (low PG score) and severe group (high PG score). DS patients with high PG score variants were no different to those with truncating variants in any of the measured seizure characteristics. In contrast low PG score variants had significantly later seizure onset compared to severe or truncating variants across all seizure types including first seizure (p<0.001), prolonged febrile (p=0.014), hemiclonic (p<0.001), myoclonic (p=0.005), GTC (p=0.012), atypical absences (p<0.001) and status epilepticus (p=0.015; table 2).Of the 50 missense variants tested experimentally, complete loss-of-function (no measurable whole-cell current) was observed in a greater proportion of DS-associated variants (54%, 13/24) compared to GEFS+ variants (24%, 4/17; p=0.050). Variants with loss-of-function effects had a higher median PG score (64, n=35) than those with “mixed effects” (46, n=10) or gain-of-function effects (30, n=3). However, numbers were too small to allow statistical comparison (p=0.083). Conclusions: The PG score combining AA position (paralog conservation) and characteristics of AA substitution can help to characterise the clinical significance of missense SCN1A variants. The clinical presentation of high PG score variants is similar to truncating variants, whereas low PG score variants are associated with less severe phenotypes. This novel approach may allow better differentiation between missense variants and prediction of disease trajectories in the future. Funding: No funding received
.tmb-.png?Culture=en&sfvrsn=6e59be10_0)