A Single-Center Experience of NORSE Over a 10-Year Period
Abstract number :
3.173
Submission category :
4. Clinical Epilepsy / 4A. Classification and Syndromes
Year :
2018
Submission ID :
502590
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Manaz Rezayee, Oregon Health and Science University; Madeline Nguyen, Oregon Health and Science University; Chad Murchison, Oregon Health and Science University; and Marissa Kellogg, Oregon Health and Science University
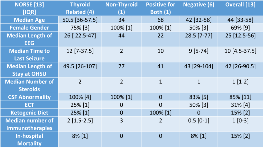
Rationale: New-Onset Refractory Status Epilepticus (NORSE) is a rare life-threatening condition in previously healthy patients who suffer from new-onset refractory status epilepticus of unclear etiology with devastating outcomes, first described in 2005. NORSE commonly leads to super-refractory status epilepticus (SRSE), a severe form of status for which seizures persist despite 24 hours of adequate anti-seizure and anesthetic treatment. While scientific literature hypothesizes that NORSE is an inflammatory autoimmune and/or post-infectious condition, the epidemiology of this rare condition remains unclear and no antibodies have been clearly associated with NORSE to date. The goal of this study is to examine the clinical characteristics of all super-refractory NORSE cases admitted to a single level IV epilepsy center over a 10-year period and to determine if the results of antibody testing are associated with outcomes. Methods: We performed an IRB-approved retrospective chart review of all cases of SRSE admitted to Oregon Health Sciences University (OHSU) between 2007 and 2016. The inclusion criteria were: 1) continuous EEG monitoring for a minimum of 4 days, 2) EEG-confirmed seizures, 3) presence of medically-induced burst suppression on EEG, 4) no pre-existing diagnosis of epilepsy, and 5) no symptomatic cause of seizures identified within 48 hours of admission. Cases were categorized into four groups by the antibody as follows: 1) positive for thyroid-related antibodies only, 2) positive for non-thyroid antibodies only, 3) positive for both thyroid and non-thyroid, and 4) negative antibody testing. Results: Among 358 patients monitored on continuous EEG for at least 4 days in a single admission, 67 (19%) of cases were identified as SRSE. Of the 67 SRSE cases, 13 (19%) of cases were identified as NORSE (an incidence of 1.3 per year at an academic tertiary care center). In-hospital mortality for NORSE was 15% (2). The median age of onset was 44 and the majority of cases (69%) were females. The average length of admission to OHSU was 42 days. Time on EEG-monitoring averaged 25 days, and time until seizure cessation was an average of 10 days. Of the 13 NORSE cases, one patient (8%) was not antibody tested. Four cases (31%) had positive anti-thyroid related (TPO or Tg) antibodies. Of the 13 cases, only one was positive for non-thyroid related (neuronal VGKC) antibodies and one was positive for both thyroid-related (TPO) and non-thyroid related (neuronal AChR) antibodies. The remaining six (46%) cases had negative antibody testing (often performed twice at separate testing centers). Conclusions: The results of this study are consistent with prior reports of NORSE epidemiology: the condition is rare, patients tend to be younger, female-predominate, and there have been no antibodies clearly-associated with the condition. However, 46% of cases had positive antibody testing – the majority of which were thyroid-associated antibodies. While thyroid-associated antibodies are thought to be non-specific markers of inflammation and/or autoimmunity, this supports the hypothesis that NORSE (or a subset of NORSE cases) may be autoimmune mediated. Funding: Build EXITO (NIH-funded research training program)
.tmb-.png?Culture=en&sfvrsn=858f65ba_0)