Analysis of Allergic Reactions in Clinical Trials of Eslicarbazepine Acetate in Children (Aged 4–17 Years) With Focal Seizures
Abstract number :
2.240
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
501737
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Trevor Resnick, Nicklaus Children's Hospital; Todd Grinnell, Sunovion Pharmaceuticals Inc.; David Cantu, Sunovion Pharmaceuticals Inc.; Yan Li, Sunovion Pharmaceuticals Inc.; Joana Graça, BIAL - Portela & C.ª, S.A.; Helena Gama, BIAL - Portela &
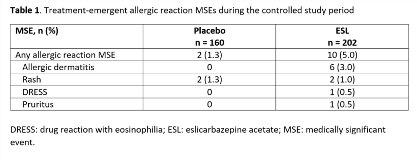
Rationale: Eslicarbazepine acetate (ESL) is a once-daily oral antiepileptic drug (AED) for focal (partial-onset) seizures (FS). Allergic reactions were reported during clinical trials of ESL in adults. Here, we evaluate the occurrence of allergic reactions in clinical trials of ESL for FS in children. Methods: Pooled data from subjects aged 4–17 yrs in Studies BIA-2093-208 and -305 were analyzed. Both trials were randomized, double-blind, placebo-controlled studies of adjunctive ESL in children with FS refractory to 1–2 AEDs. Study 208-Part 1 was a 12-week, Phase II study in subjects aged 6–16 yrs, with a target ESL dose of 30 mg/kg/day. Study 305-Part 1 was an 18-week, Phase III study in subjects aged 2–18 yrs, with a target ESL dose of 20 mg/kg/day. Subjects could continue into a 1-yr uncontrolled, open-label extension (OLE) in both studies (Part 2), and into further OLEs (Study 208, Part 3; Study 305, Parts 3–5). We evaluated incidences of allergic reaction medically significant events (MSEs) i.e., investigator-reported treatment-emergent adverse events (TEAEs) that included ‘blister*’, ‘prurit*’, ‘rash’, ‘exanthema’, ‘erythema*’, ‘rosacea’, ‘urticaria’, or ‘dermatitis’, were in the standardized MedDRA queries (SMQs) of ‘severe cutaneous adverse reactions’, ‘angioedema’, ‘allergic conditions not elsewhere classified (NEC)’, or ‘anaphylactic reaction’, or were in the ‘angioedema’, ‘urticaria’, or ‘allergic conditions NEC’ categories, and were fatal, serious, severe, led to discontinuation, required other action/treatment, or were otherwise significant. Drug reaction with eosinophilia and systemic symptoms (DRESS) identified via RegiSCAR items, signs, and symptoms was also considered an MSE. Results: The safety population (subjects aged 4–17 yrs who received =1 dose of study drug) for the controlled study period included 362 subjects (ESL, n = 202; placebo, n = 160); 337 continued into the OLE. The overall incidences of allergic reaction MSEs during the controlled studies were 5% and 1.3% with ESL and placebo, respectively (Table 1). The most frequently reported allergic reaction MSEs were allergic dermatitis and rash (Table 1). There were no cases of Stevens-Johnson Syndrome (SJS) or Toxic Epidermal Necrolysis (TEN). Eight subjects had MSEs that were non-serious, mild-moderate in severity, and did not lead to discontinuation. Six subjects had allergic reaction MSEs that were classified as non-serious but required treatment. There were three non-serious allergic reaction MSEs (rash, n = 1; allergic dermatitis, n = 2), and one investigator-reported case of serious DRESS, that led to discontinuation of ESL. Nine subjects had an allergic reaction MSE during the uncontrolled OLEs (Table 2). Conclusions: Allergic reactions characterized as MSEs occurred in 5% of children aged 4–17 years during double-blind treatment with adjunctive ESL for FS, and were generally non-serious. There was one case of DRESS, and no cases of SJS or TEN. Funding: Studies sponsored by BIAL; analyses funded by Sunovion Pharmaceuticals Inc.
.tmb-.png?Culture=en&sfvrsn=5eab47fd_0)