Behavioral and Cognitive Effects of Long-Term Adjunctive Brivaracetam in Children With Epilepsy
Abstract number :
1.292
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
497206
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Lieven Lagae, University of Leuven; Teresa Gasalla, UCB Pharma; Simon Borghs, UCB Pharma; Melinda Martin, UCB Pharma; Jody Cleveland, UCB Pharma; Vincent Badalamenti, UCB Pharma; Jan-Peer Elshoff, UCB Pharma; and Jesus E. Piña-Garza, Tristar Medical
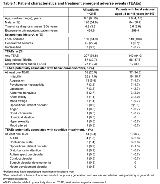
Rationale: This pooled interim analysis investigated behavioral and cognitive effects of long-term adjunctive brivaracetam (BRV), which is an important consideration for antiepileptic drug (AED) treatment in children with epilepsy. Methods: N01263 (NCT00422422) was an open-label trial of adjunctive BRV in children (≥1 month to <16 years) with epilepsy uncontrolled by 1-3 concomitant AEDs. BRV dose was up titrated over 3 weeks (≥8 years: 0.4, 0.8, 1.6 mg/kg bid; <8 years: 0.5, 1.0, 2.0 mg/kg bid). Patients who completed the dose-escalation period could continue on BRV in an open-label extension (N01266; NCT01364597), which also enrolled new patients (≥4 to <17 years) with uncontrolled focal seizures. After N01266 dose-adjustment, patients received BRV at a dose of 1-5 mg/kg/day (max 200 mg/day). This pooled interim analysis (cut-off: March 15, 2017) investigated changes from N01266 Baseline in the Behavior Rating Inventory of Executive Function (BRIEF; 5-16 years) and Achenbach Child Behavior Checklist (CBCL; 6-18 years) at 2, 12, and 24 months, and treatment-emergent adverse events (TEAEs) potentially related to behavior and cognition. Results: Data were obtained from 219 patients, 149 of whom were aged ≥4 to <16 years with focal seizures (focal 4-16) (Table 1). At the cut-off date, 118 (53.9%) patients were ongoing in N01266, 97 (44.3%) had discontinued the trial, and 4 (1.8%) had chosen not to enter N01266. Overall exposure to BRV was 464.6 patient-years (focal 4-16: 299.4 patient-years). For the BRIEF (5-16 years; n=104), decreases (improvements) in T-scores from Baseline to 2, 12, and 24 months were observed for the Global Executive Composite score (range: -2.0 to -3.0), Behavioral Regulation Index (-1.7 to -3.0), and Metacognition Index (-2.0 to -2.8). Mean changes from Baseline in CBCL T-scores (6-18 years; n=129) showed small decreases (improvements) at 2, 12, and 24 months for all subscales (range: -0.1 to -5.3), except Withdrawn/Depressed (0.1 to 0.6). For both BRIEF and CBCL subscales, most patients were stable with no shifts from Baseline to Last Value in T-score categories (normal/borderline/clinically significant) (Table 2). Incidences of TEAEs were similar in the overall population and focal 4-16 group (Table 1). In the overall population, 59 (26.9%) patients had a behavioral TEAE, most commonly irritability (26 [11.9%]) and aggression (13 [5.9%]). Behavioral TEAEs were most common during the first 3 months of treatment. Most events were mild or moderate in intensity and did not lead to discontinuation; 2 patients had a severe event (aggression; laceration). TEAEs potentially related to cognition were reported by 14 (6.4%) patients, most commonly ADHD (4 [1.8%]) and confusional state (3 [1.4%]). Conclusions: In children on long-term adjunctive BRV, measures of behavior and cognition were generally stable over time and suggest a limited impact on behavior and no impact on cognition. Behavioral TEAEs were more commonly experienced early in therapy, and few patients reported cognitive TEAEs. Further investigations are needed to confirm these results. Funding: UCB Pharma-sponsored
.tmb-.png?Culture=en&sfvrsn=201fab58_0)