BIOAVAILABILITY AND TOLERABILITY OF RECTALLY ADMINISTERED OXCARBAZEPINE SUSPENSION
Abstract number :
2.348
Submission category :
Year :
2005
Submission ID :
5655
Source :
www.aesnet.org
Presentation date :
12/3/2005 12:00:00 AM
Published date :
Dec 2, 2005, 06:00 AM
Authors :
1Pamela L. Clemens, 1James C. Cloyd, 2Robert L. Kriel, and 1Rory P. Remmel
Rectal administration of some AEDs is a viable alternative when the oral route is temporarily unavailable. Oxcarbazepine (OXC) is a newer AED that is rapidly converted to a monohydroxy derivative (MHD), the active compound. Our study was designed to characterize the bioavailability and tolerability of OXC suspension (300 mg/5 ml) administered rectally. This study used a randomized, crossover design in 10 healthy volunteers who were randomized to the order of routes of administration. Subjects fasted the morning of each dose and administered a Fleet[apos]s enema before the rectal dose. The rectal dose was mixed with an equal volume of water and administered via a catheter attached to a syringe. A two-week wash-out occurred between doses.
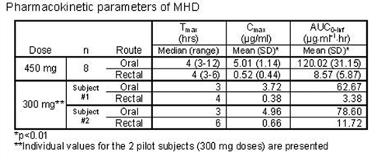
Blood was drawn pre-dose and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, 48, and 72 hours post-dose. The first 2 subjects received 300 mg doses. Following review of concentrations and tolerability, the remaining 8 subjects received 450 mg doses. Side effects were assessed at each sampling timepoint using a self-administered questionnaire with a 5-point rating scale (5 = most severe).
Plasma samples were assayed for OXC and MHD using a validated HPLC assay. Lower limit of detection for MHD was 0.01 mcg/ml and 0.015 mcg/ml for OXC. Maximum concentration (C[sub]max[/sub]) and time to maximum concentration (t[sub]max[/sub]) were obtained directly from the plasma concentration-time curves (AUCs). AUCs were determined for both OXC and MHD via non-compartmental analysis using WinNonLin. Relative bioavailability was calculated as (AUC[sub]rectal[/sub] / AUC[sub]oral[/sub])*100%. AUC and C[sub]max[/sub] of MHD following each route of administration were compared using Wilcoxon signed-rank tests. Mean relative bioavailability of MHD was 10.15% (SD 6.73) in the 2 subjects receiving 300 mg doses and 7.77% (SD 5.93) in the 450 mg dose subjects. The MHD C[sub]max[/sub] and AUC at the 450 mg dose level differed significantly between routes (p[lt]0.01).[figure1]The most common side effects were headache and fatigue; there was no discernable difference between routes. One subject rated discomfort of rectal administration as 3/5, another reported discomfort of oral administration 1/5 due to taste. Most subjects had a few clinical labs outside the normal range, each was considered clinically insignificant. The C[sub]max[/sub] and exposure to MHD after rectal administration of OXC suspension are significantly less than after oral administration. It is unlikely that adequate MHD concentrations can be reached by rectally administering diluted OXC suspension. (Supported by Novartis [amp] University of Minnesota.)