Cannabinoid Knowledge and Attitudes among US Healthcare Providers
Abstract number :
3.469
Submission category :
17. Public Health
Year :
2018
Submission ID :
555794
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Magdalena Szaflarski, University of Alabama at Birmingham; Patricia McGoldrick, Icahn School of Medicine at Mount Sinai; Lauryn Currens, St. George's University School of Medicine; Dustin Blodgett, St. George's University School of Medicine; Hunter Land,
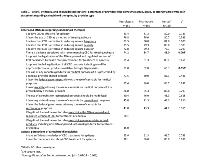
Rationale: Cannabinoids are gaining acceptance for the management of treatment-resistant epilepsy (TRE). Policy changes and recent approval of Epidiolex® (cannabidiol [CBD]) for seizures associated with Lennox-Gastaut and Dravet syndromes have exposed providers to questions from patients/caregivers regarding the appropriateness of cannabinoids for TRE. We compared attitudes toward and knowledge of cannabinoid therapies among different provider types. Methods: Targeted, national, and quota-based online survey of US neurologists (n=151), pharmacists (n=150), and nurses (n=150 [60 NPs]) was conducted in August-September 2018 after FDA approval of Epidiolex®. 29 structured items tapped attitudes toward cannabinoid therapies, knowledge of endocannabinioid system, pharmacology, FDA vs non-FDA approved products, effects, clinical application, and regulation. Respondent’s demographics, work setting, pediatric/adult patient distribution, distribution of seizure disorders, and organizational policy regarding administration of cannabis-derived products were assessed. Analysis was based on cross-tabulations and Chi-square tests. Results: Respondents were 53% female, 83% white, and 4% Hispanic/Latino. 40% worked in private practice and 24% in academic hospitals. Differences in responses based on provider type were largely nonsignificant (p≥0.188; Tables 1-2). 80-83% per group believed that CBD is effective for epilepsy and 76-81% favored use of CBD for TRE (Table 1). 74%-88% agreed that there is a stigma associated with recommending CBD for TRE, though neurologists were least likely to agree (p=0.005). 89-91% supported legalization of FDA-approved CBD treatment prescribed by health care professionals, but less so legalization of all CBD products (51% neurologists vs 62-65% pharmacists/nurses, p=0.030). 85-88% favored the federal government and their state allowing use of cannabis for medical purposes. 61-66% agreed that they have sufficient knowledge of CBD treatment for TRE, but 78-86% indicated a need for further education. Actual knowledge of cannabis-based products was mixed (Table 2): only 30-34% per group knew how many different phytocannabinoids are present in the cannabis plant, but 81-88% agreed that effects of cannabis-derived products depend on cannabinoid content. Neurologists were most likely (p=0.001) to correctly answer about testing serum levels of AEDs, but overall few providers (5%-16%) answered correctly. Pharmacists were most likely (p≤0.021) to correctly state that hemp is legal (59%) and cannabis is not (81%); only 38% and 75% of neurologists answered these questions correctly. 31-42% of providers across groups stated that isolated plant-derived cannabinoids are legal. Conclusions: US providers appear to believe in beneficial effects and support legalization of CBD for treatment of TRE. Notable knowledge gaps were present in questions related to legality and potential interactions with AEDs. The majority think that they have sufficient knowledge of cannabis/cannabis-based therapies, but overwhelmingly indicate a need for further education. Funding: Greenwich Biosciences, Inc.
.tmb-.jpg?Culture=en&sfvrsn=3114e5bf_0)