Cinematic Projection of Ictal Activity on Three-Dimensional Brain Surface Renderings as an Adjunct for Seizure Localization
Abstract number :
3.122
Submission category :
3. Neurophysiology / 3A. Video EEG Epilepsy-Monitoring
Year :
2018
Submission ID :
502610
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Jonathan K. Kleen, UCSF Medical Center; Maxime R. Baud, University Hospital Bern; Benjamin Spiedel, UCSF Medical Center; Simon Ammanuel, UCSF Medical Center; Liberty Hamilton, University of Texas at Austin; Edward F. Chang, UCSF Medical Center; and Robert
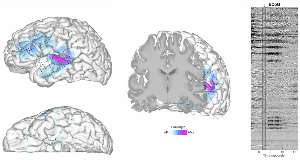
Rationale: Human intracranial electrocorticography (ECoG) recordings provide unparalleled perspectives for direct seizure localization. However, they are often cumbersome to interpret, involving the clinically-directed placement of hundreds of electrodes in various locations, rendering as many data traces with complex labeling (e.g. grid vs. strip vs. depth sites). Thus, from patient to patient, spatial coverage is highly variable and individual anatomical differences further cloud the spatial origins of signal sources. Digital brain reconstructions offer patient-specific visualization of anatomy and implanted electrode locations, and enable colorization of regional functional activity. We used these techniques to visualize peri-ictal ECoG activity on patient-specific brain surfaces (digitally in situ) of 8 patients that had undergone ECoG monitoring at our center, and compared visually-detected seizure-onset zones (SoZs) to actual clinically-determined SoZs. Methods: Eight patients with medically-refractory seizures underwent intracranial implantation of grid, strip and depth electrodes (range: 96 to 156 contacts per patient). All patients had a pre-operative MRI and post-operative CT for co-registration of electrode locations, and this data was channeled through our in-house research pipeline combined with Freesurfer (Charlestown, MA) for detailed cortical surface renderings. ECoG data was transformed using a sliding line length calculation with a 250 ms window. This quantity was colorized as a functional projection onto the 3-D brain surface, allowing visualization of activity from the original anatomic sources. MRI voxel data was also utilized to create 3-D slice views, enabling concurrent visualization of depth electrode activity in deep regions. Peri-ictal data was converted into videos with multiple 3-D brain views and unlabeled ECoG waveform data adjacently. Five people of varying expertise in epilepsy (1 graduate student, 1 neurology resident, 2 epilepsy fellows, and 1 fully trained epileptologist) viewed these videos in an attempt to localize the SoZ, and these localizations were compared to the original clinically-determined SoZ. Results: Line length projections onto the brain surface and deep slices enabled visualization of relative inter-electrode differences, with emphasis on sharp and large-amplitude activity to accentuate epileptiform features. Compared to the clinically-determined SoZ, the line length projection-based SoZ was accurately determined for 7 out of 8 patients among attending and fellow-level scorers (N=3). Resident and student level scorers were highly concordant, accurately identifying 6 of 8 cinematic-based SoZs (N=2). Using shorter line length windows (40ms), interictal spike source and propagation patterns were determined consistently for all patients (8/8), aligning with their clinical reports. Conclusions: We present an intuitive open-source method for clinicians with (and potentially without) formal epilepsy training to localize SoZs. Concordance was relatively high, though interpretability was likely attenuated by activity color scale calibration and selective viewing angles. We are currently expanding this study for more formal analysis of efficacy, and to determine whether this method can help identify non-resected sites of activity that may predict any return of seizures after surgery. This preliminary study is encouraging for the potential use of this method as an adjunct for the interpretation of anatomic regions implicated in focal epilepsy, and as an education tool for trainees at all levels. Funding: Dr. Kleen: NINDS (R25NS070680-07), Dr. Chang: NIH (R01-DC012379, R00-NS065120), DARPA (DP2-OD00862), Esther A. and Joseph Klingenstein Fund
.tmb-.png?Culture=en&sfvrsn=5f93ff94_0)