Feasibility of Guiding sEEG Implantation in Focal Epilepsy Using Automated Surface-Based Analysis and Machine Learning
Abstract number :
1.251
Submission category :
5. Neuro Imaging / 5A. Structural Imaging
Year :
2018
Submission ID :
499342
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Konrad Wagstyl, Brain Mapping Unit, University of Cambridge; Sophie Adler, UCL Great Ormond Street Institute of Child Health; Birgit Pimpel, UCL Great Ormond Street Institute of Child Health; Sara Lorio, UCL Great Ormond Street Institute of Child Health;
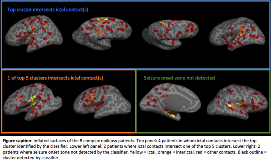
Rationale: Stereoencephalography (sEEG) is a technique for recording intracranial EEG in which multiple depth electrodes are stereotactically placed within the brain to identify seizure onset zone. It is an invasive study typically conducted on patients with drug-resistant epilepsy with the aim to capture network distribution involved in seizure generation and propagation. The number and location of implanted electrodes is clinically driven. However, with complex patients, this can often result in over 10 electrodes being implanted. We have previously demonstrated the ability of surface-based MRI analysis and machine-learning to automatically detect focal lesions (Adler, Wagstyl et al., NICL, 2017; Jin et al., Epilepsia, 2018). Here we investigate the potential of this method to target sEEG implantation. Methods: The MRI and sEEG data of a retrospective cohort of 8 paediatric patients, who underwent invasive sEEG monitoring due to inconclusive non-invasive investigations, and had a focal, cortical seizure onset as defined by sEEG, were reviewed. A neural network classifier was trained on surface-based data from 34 patients with visible focal cortical dysplasias (FCDs) using the freely available methods previously published by our group. The trained network was tested on the MRI data of the 8 sEEG patients. The output probability maps from the classifier were grouped into neighbour-connected clusters and the 5 clusters with the highest mean probability values were evaluated. The coordinates of the sEEG contacts were coregistered to the nearest cortical surface in native space. sEEG contacts involved in ictal and inter-ictal activity were identified by clinical neurophysiologists. The relationship between the automated clusters detected by the classifier and ictal sEEG contacts was assessed. Results: In 6 out of 8 patients (75%) there was a close association between the location of ictal sEEG contacts and a cluster detected by the automated classifier (Figure). In 4 out of 8 patients (50%) the top cluster, i.e., the area considered most likely to be lesional by the classifier, overlapped an ictal contact, whereas in 2 patients one of the top five clusters overlapped the seizure onset zone. In 2 patients the classifier was unable to detect the seizure onset zone defined by sEEG. Conclusions: This preliminary, retrospective study suggests that surface-based MRI features incorporated into a neural network can detect focal MRI abnormalities in proximity of the sEEG-determined seizure onset zone in 6 out 8 patients. Automated surface-based analysis and machine learning may be a new way to target sEEG implantation in epilepsy patients. In the future, this could reduce the number of electrodes implanted, reducing the risks of the invasive procedure. Furthermore, it may be useful in optimising electrode placement in MRI negative patients, as well as reducing the number of unsuccessful sEEGs which do not delineate the seizure onset zone. Funding: We thank the Rosetrees Trust for their generous support.