GIRK2 CHANNEL-TARGETED RESCUE STRATEGIES FOR INFANTILE SPASMS
Abstract number :
2.386
Submission category :
Year :
2014
Submission ID :
1868938
Source :
www.aesnet.org
Presentation date :
12/6/2014 12:00:00 AM
Published date :
Dec 4, 2014, 06:00 AM
Authors :
O. Carter Snead, M.D., Krutika Joshi, B.Sc., Miguel Cortez, M.D., James Eubanks, Ph.D., Avner Michaeli, B.Sc. and Michael Salter, M.D., Ph.D.
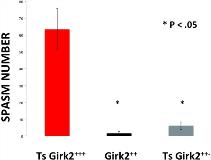
Rationale: Infantile spasms (IS) is a catastrophic childhood epilepsy that is prevalent in children with Down syndrome. γ-aminobutyric acid(B) receptor (GABABR) agonists can induce an IS phenotype in the Ts65Dn (Ts) mouse model of Down syndrome. The G-protein-coupled inward rectifying potassium channel subunit 2 (GIRK2) gene is over-expressed (Ts65Dn GIRK2+/+/+) and the GABABR-mediated GIRK2 current significantly increased in Ts. We hypothesized that the GIRK2 channel is necessary and sufficient for the IS phenotype induced in the Ts mice by GABABR agonists. We tested this hypothesis by ascertaining the EEG and behavioral effects of a GABABR agonist in Ts where GIRK2 was knocked down either pharmacologically or genetically. Methods: Pharmacological experiments: We reduced the GIRK2 channel activity in the Ts mice pharmacologically using a general GIRK 1-4 antagonist tertiapin Q (TPQ) which was administered intracerebroventricularly (ICV) to Ts mice prior to administration of γ-butyrolactone (GBL), the prodrug of γ-hydroxybutyric acid (GHB), a GABABR agonist that we have shown to induce an IS phenotype in Ts. Genetic experiments: We used GIRK2-/- mice to engineer Ts mouse disomic for the GIRK2 gene (Ts65Dn GIRK2+/+/-) and determined the EEG and behavioral effect of GBL in the disomic Ts mutant. Molecular confirmation of the GIRK2 mutation was done all mutant animals by genotyping. IS phenotype determination. EEG and behavioral responses to GBL were observed and quantitated as described (Cortez et al. Pediatr Res 2009;65,499) in Ts GIRK2 disomic and TPQ treated animals with appropriate pharmacologic and genetic controls. Electrophysiology: Whole-cell potentials were recorded from CA1 pyramidal neurons in hippocampal slices prepared from the Ts65Dn GIRK2+/+/- mutant and all genetic controls. The ratio between GABABR-mediated IPSP and total EPSP was determined to assess GABABR function. Results: Pharmacological antagonism of GIRK channels in Ts with TPQ resulted in marked resistance to the GABABR-induced IS phenotype in Ts. The GABABR-induced IS phenotype was abolished in the Ts mouse made disomic for the GIRK2 gene (Ts65Dn GIRK2+/+/-) (Fig.). GABABR currents were increased in Ts65Dn GIRK2+/+/+ vs wild type controls (GIRK2+/+) and Ts65Dn GIRK2+/+/-. There was no difference in GABABR currents between Ts65Dn GIRK2+/+/- and GIRK2+/+. Conclusions: In addition to opening up entire new avenues of investigation for the pathogenesis of IS, our data suggest that the GIRK2 channel in brain may present a novel therapeutic target for IS. As well, these data are highly significant in view of successful targeting of the potassium channel, KCNT1 in the treatment of yet another catastrophic childhood epilepsy, migrating partial seizurs of infancy (Bearden D, et al. Ann Neurol 2014;76:457).